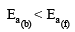PYQ Test: Thermodynamics(26 Feb) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - PYQ Test: Thermodynamics(26 Feb)
The heat required to raise the temperature of body by 1 K is called [2002]
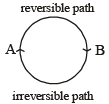
The internal energy change when a system goes from state A to B is 40 kJ/mole. If the system goes from A to B by a reversible path and returns to state A by an irreversible path what would be the net change in internal energy ? [2003]
If at 298 K the bond energies of C — H, C — C, C = C and H — H bonds are respectively 414, 347, 615 and 435 kJ mol–1, the value of enthalpy change for the reaction H2C = CH2(g) + H2(g) → H3C — CH3(g) at 298 K will be [2003]
In an irreversible process taking place at constant T and P and in which only pressure-volume work is being done, the change in Gibbs free energy (ΔG) and change in entropy (dS), satisfy the criteria [2003]
The correct relationship between free energy change in a reaction and the corresponding equilibrium constant Kc is [2003]
The enthalpy change for a reaction does not depend upon [2003]
An ideal gas expands in volume from 1×10–3 to 1 × 10–2 m3 at 300 K against a constant pressure of 1×105 Nm–2. The work done is [2004]
The enthalpies of combustion of carbon and carbon monoxide are – 393.5 and – 283 kJ mol–1 respectively. The enthalpy of formation of carbon monoxide per mole is
Consider an endothermic reaction X → Y with the activation en er gies Eb and Ef for the backward and forward reactions, respectively. In general [2005]
Consider the reaction : N2 + 3H2 → 2 NH3 carried out at constant temperature and pressure. If ΔH and ΔU are the enthalpy and internal energy changes for the reaction, which of the following expressions is true ? [2005]
|
360 tests
|



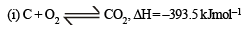
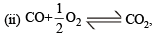
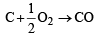 ΔH = -110.5 kJmol–1
ΔH = -110.5 kJmol–1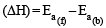 for an endothermic reaction ΔH = +ve hence for ΔH to be negative
for an endothermic reaction ΔH = +ve hence for ΔH to be negative