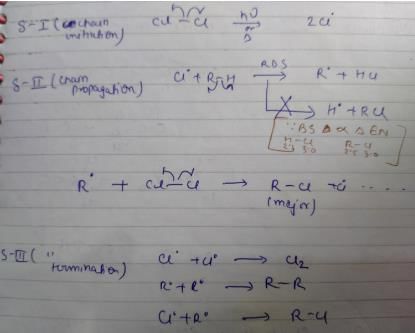Practice Test - NEET MCQ
30 Questions MCQ Test 4 Months Preparation for NEET - Practice Test
The amount of work done in moving a charge from one point to another along an equipotential line or surface charge is
In bringing an electron towards another electron, the electrostatic potential energy of the system
The potential energy of a system containing only one point charge is
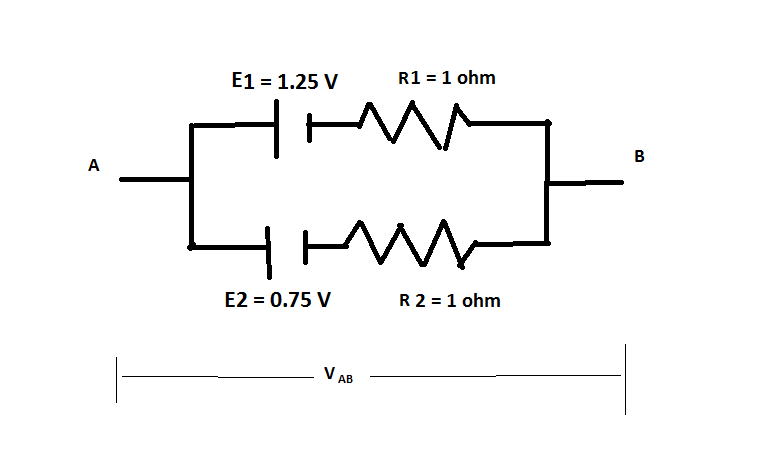
Two cells of emf 1.25V , 0.75V and each of internal resistance 1Ω are connected in parallel. The effective emf will be
Which of the following is not a possible termination step in the free radical chlorination of methane?
The major monobromination product which results when ethyl cyclohexane is subjected to free radical bromination, is
What is relative reactivity of secondary versus primary hydrogens in free radical bromination of n-butane if the ratio of 1-bromo to 2-bromobutane formed is 7 : 39?
What is the major monobromination product in the following reaction?
Sickle cell anemia has not been eliminated from the African population because
Which of the following disease is caused by virus and transmitted by mosquito?
Cancer detection is not based on which of the following tests or studies?
The primary and secondary immune response are carried out with the help of two special types of lymphocytes present in our blood called?
An insect bite may result in inflammation of that spot. This is triggered by the alarm chemicals such as
Anti-venom is used for the treatment of snake bite. The treatment of snake bite by anti-venom is an example of
Which of the following gas is responsible for the puffed-up appearance of the dough?
Consider the following statements about the lab.
- It increases the vitamin B12 amount, thus increasing the nutrient quality of milk.
- It checks disease-causing microbes in the stomach.
Choose the correct option.
Assertion (A): yeasts such as saccharomyces cerevisiae are used in the baking industry.
Reason (R): Carbon dioxide produced during fermentation causes bread dough to rise by thermal expansion.
|
440 videos|1595 docs|542 tests
|