Practice Test - NEET MCQ
30 Questions MCQ Test 4 Months Preparation for NEET - Practice Test
Which of the following statements about earth's magnetism is correct
A bar magnet of magnetic moment 1.5 J/T lies aligned with the direction of a uniform magnetic field of 0.22 T. What is the amount of work required by an external torque to turn the magnet so as to align its magnetic moment opposite to the field direction?
In the magnetic meridian of a certain place, the horizontal component of the earth’s magnetic field is 0.26G and the dip angle is 60o. What is the magnetic field of the earth at this location
A cube-shaped permanent magnet is made of a ferromagnetic material with a magnetization 500M of about The side length is 20 cm. Magnetic dipole moment of the magnet is.
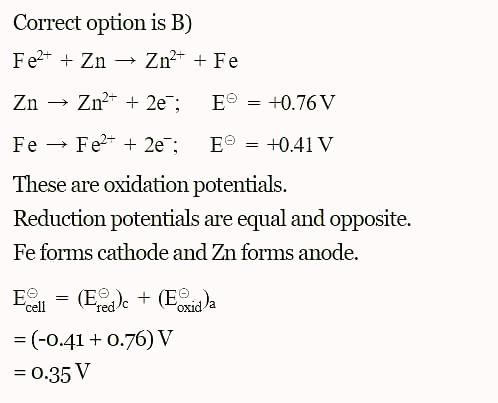
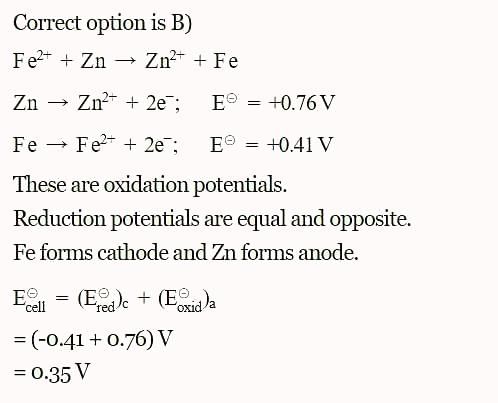
The standard reduction potential at 298 K for the following half cells are given:
Which is the strongest reducing agent:
Which of the following conditions are satisfied when the cell reaction in the electrochemical cell is spontaneous?
What is the observation when the opposing external applied potential to an electrochemical cell is greater than the cell’s potential?
The standard reduction potentials E°, for the half reactions are as:
The emf for the cell reaction,
In agarose gel electrophoresis, DNA molecules are separated on the basis of their:
Origin of replication is the specific DNA sequence on chromosome that which is responsible for:
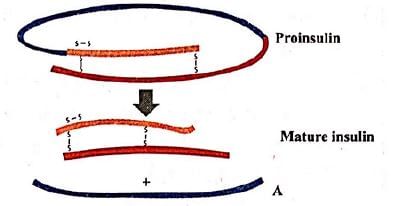
Which of the following statements regarding the structure of proinsulin and mature insulin are not correct?
(i) Proinsulin is made up of three polypeptide chains- A, B and C.
(ii) C - polypeptide chain with 33 amino adds is removed prior to insulin formation.
(iii) Mature insulin is made up of 51 amino acids arranged in two polypeptide chains- A and B.
(iv) Polypeptide chain A has 30 amino acids and polypeptide chain B has 21 amino acids.
(v) Polypeptide chains A and B are interconnected by only one S−S linkage.
Which of the following statements regarding gene therapy is/are correct?
Read the given statements and select the correct option
Statement 1: PCR technique is helpful in detecting bacterial and viral diseases even when symptoms of the disease are not yet visible
Statement 2: Very low concentrations of bacteria or viruses in human body can be detected by amplification of their nucleic acids using the PCR technique
Given figure represents the maturation of pro-insulin into insulin. Identify the product A.
What might be an advantage of beginning gene therapy prior to birth?
|
440 videos|1595 docs|542 tests
|