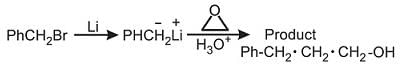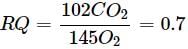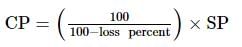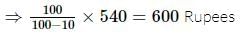SRMJEE Mock Test - 4 (Medical) - JEE MCQ
30 Questions MCQ Test - SRMJEE Mock Test - 4 (Medical)
Which of the following show(s) the particle and wave nature of electromagnetic waves and electrons?
Two point charges 3 × 10-6 C and 8 × 10-6 C repel each other by a force of 6 × 10-3 N. If each of them is given an additional charge of -6 × 10-6 C, then the force between them will be
If h is Planck's constant, the momentum of a photon of wavelength 0.01 Å is
Which of the following rules is applicable on an electric motor?
A particle of mass m at rest is acted upon by a force P for a time t. Its kinetic energy after an interval t is
A gas under constant pressure of 4.5 × 105 Pa, when subjected to 800 kJ of heat, changes the volume from 0.5 m3 to 2.0 m3. The change in internal energy of the gas is:
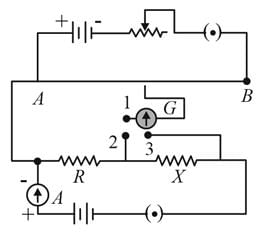
A potentiometer circuit is set up as shown. The potential gradient across the potentiometer wire, is k volt/cm and the ammeter, present in the circuit, reads 1.0 A when two way key is switched off. The balance points, when the key between the terminals (i) 1 and 2 (ii) 1 and 3, is plugged in, are found to be at lengths l1 cm and l2 cm respectively. The magnitudes, of the resistors R and X, in ohms, are then, equal, respectively, to
[Given: Ebullioscopic (molal boiling point elevation) constant of water, Kb = 0.512 K Kg mol-1]
Thermal decomposition of N2O5 occurs as per the equation below:
2N2O5 → 4NO2 + O2
The correct statement is
Which one of the following aqueous solutions will exhibit the highest boiling point?
Which of the following has the highest melting point?
Select the compound which on treatment with nitrous acid liberates nitrogen.
Among the following, the incorrect statement about colloids is:
When CH2 = CH−COOH is reduced with LiAlH4, the compound obtained will be
The number of unidentate ligands in the complex ion is called
Which statement does not apply to SO₂Cl₂ and SOCl₂?
Select the method that could be used to prepare the compound PhCH2CH2CH2OH
Which of the following is a characteristic feature of sponges?
What is the defining characteristic of renal calculi?
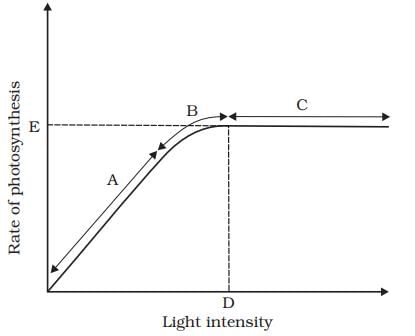
Given a graph with light intensity on the x-axis and photosynthesis rate on the y-axis, at what point does the rate of photosynthesis stop increasing with increased light intensity due to other limiting factors?
Read the following four statements carefully.
(i) Asthma is a difficulty in breathing causing wheezing due to inflammation of bronchi and bronchioles.
(ii) The part of the respiratory system starting with the external nostrils up to the terminal bronchioles constitutes the respiratory or exchange part of the respiratory system.
(iii) During swallowing epiglottis can be covered by a thin elastic cartilaginous ilap calied glottis to prevent the entry of food into the larynx.
(iv) Binding of oxygen with haemoglobin is primarily related to partial pressure of O2. Which of the above two statements are correct?
Refer the given equation.
2(C51H98O6) + 145O2 → 102CO2 + 98H2O + Energy
The RQ in this case is
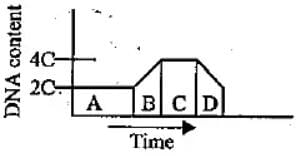
The given graph shows the change in DNA content during various phases (A to D) in a typical mitotic cell cycle. Identify the phases and select the correct option.
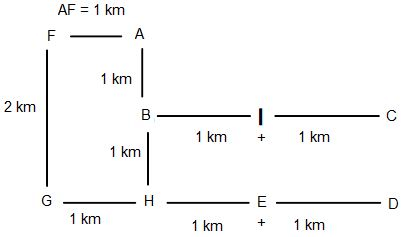
Directions: Study the following information to answer the given question.
A, B, C, D, E, F, G, H and I are nine houses. C is 2 km to the east of B. A is 1 km to the north of B and H is 2 km to the south of A. G is 1 km to the west of H while D is 3 km to the east of G and F is 2 km to the north of G. I is situated just in the middle of B and C while E is just in the middle of H and D.
Distance between A and F is
A man sold a fan for 540 Rs losing 10% ( Loss of 10% ). At what price should he have sold it to gain 10%?
How many such pairs of letters are there in the word SEARCHES, each of which has as many letters between them in the word as in the English alphabet?
Choose the sentence which is nearest in meaning to the given phrases.
Bad Blood


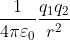 N
N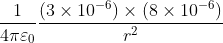 ...(i)
...(i)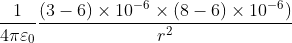
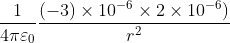 N ... (ii)
N ... (ii)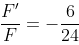
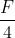
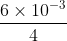 = - 1.5 × 10-3 N
= - 1.5 × 10-3 N
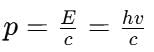
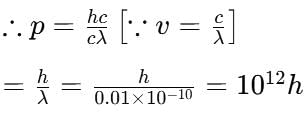
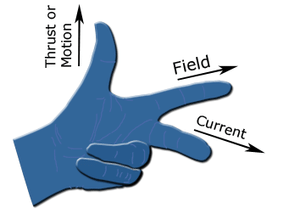
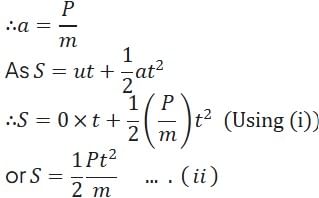
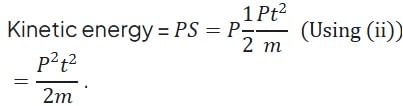
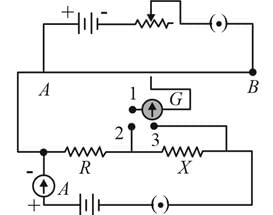
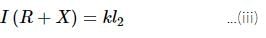
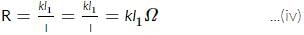
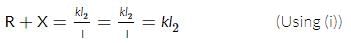
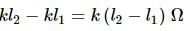 (Using (iv))
(Using (iv))
 Tb = i.Kb . m = 2 × 0.512 × 0.1 = 0.1024
Tb = i.Kb . m = 2 × 0.512 × 0.1 = 0.1024 = 100 + 0.1024 = 100.10
= 100 + 0.1024 = 100.10 




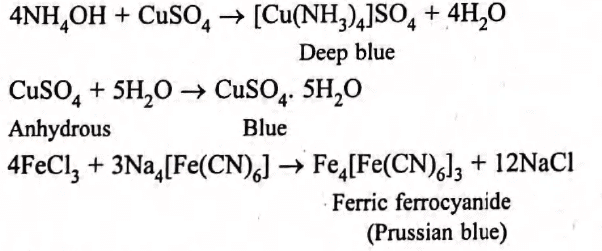
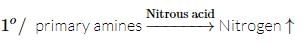
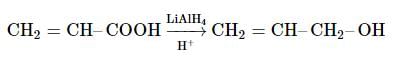
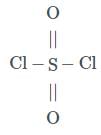
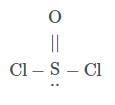 a
a