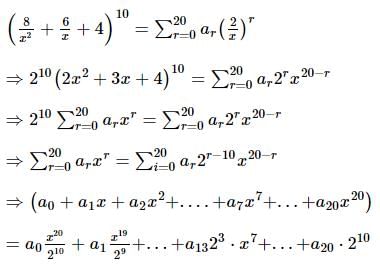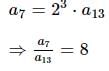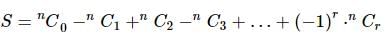SRMJEE Mock Test - 5 (Engineering) - JEE MCQ
30 Questions MCQ Test - SRMJEE Mock Test - 5 (Engineering)
A wire can be broken by applying a load of 200 N. The force required to break another wire of the same length and the same material, but double in diameter, is
An infinite number of charges, each equal to q, are placed along the x-axis at x = 1, x = 2, x = 4, x = 8, and so on. The electric field at point x = 0 is
Ultraviolet radiation of energy 6.2 eV falls on the surface of aluminium of work function 4.2 eV. What will be the K.E. of the fastest electron (in joule)?
The temperature of equal masses of three different liquids A, B, and C are 12℃, 19℃, and 28℃, respectively. The temperature when A and B are mixed is 16℃, and when B and C are mixed is 23℃. The temperature when A and C are mixed is:
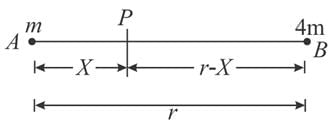
Two bodies of masses m and 4m are placed at a distance r. The gravitational potential at a point on the line joining them where the gravitational field is zero is
A pendulum has time period T in air. When it is made to oscillate in water, it acquires a time period, T' = √2T. The density (in g cm−3) of the pendulum bob is (density of water = 1 g cm−3)
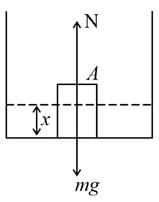
A cube of density 0.5 g cm−3 is placed in vessel and a liquid of density 1 g cm−3 is gradually filled in the vessel at a constant rate then, the graph representing the variation of normal reaction of vessel on cube and time is
The electric field of an electromagnetic wave traveling through a vacuum is given by the equation E = E₀ sin(kx − ωt). The quantity that is independent of wavelength is:
The specific conductance (K) of 0.02 M aqueous acetic acid solution at 298 K is 1.65 × 10-4 S cm. The degree of dissociation of acetic acid is
[Given, equivalent conductance at infinite dilution of H+ = 349.1 S cm2 mol-1 and CH3COO- = 40.9 S cm2 mol-1]
Directions: In the following question, two statements are given. One is assertion and the other is reason. Examine the statements carefully and mark the correct answer according to the instructions given below.
Assertion: Resorcinol turns FeCI3 solution purple.
Reason: Resorcinol contains phenolic groups.
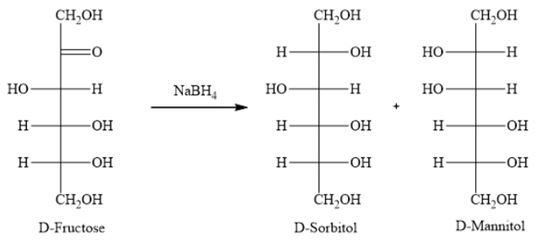
Which of the following, on reduction with NaBH4, gives an equimolar mixture of sorbitol and mannitol?
The α−amino acid which doesn't give purple colour in the ninhydrin test is
What will be the effect of acidity on activity of ptyalin enzyme in stomach?
In a hydrogen-oxygen fuel cell, the combustion of hydrogen occurs to
Following statements regarding the periodic trends of chemical reactivity of the alkali metals and the halogens are given. Which of these statements gives the correct picture?
Substance used for bringing down temperature in high fever is called
If α and β are the roots of the equation x2 - 2x - 1 = 0, the value of (α2 + β2) is
If (√3 + i)10 = a + bi; a, b ∈ R, then a and b respectively are
The probability that a marksman will hit a target is given as 1/5. Then, the probability of at least one hit in 10 shots is
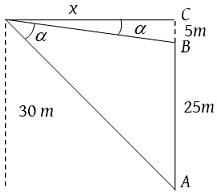
A flagstaff of 5 m high stands on a building of 25 m high. At an observer at a height of 30 m. The flagstaff and the building subtend equal angles. The distance of the observer from the top of the flagstaff is:
If a, b, and c are in geometric progression and the roots of the equation ax² + 2bx + c = 0 are α and β, and the roots of the equation cx² + 2bx + a = 0 are γ and δ, then:
The function f:R→R defined by f(x) = (x − 1)(x − 2)(x − 3) is
If nC0 - nC1 + nC2 - nC3 + ... + (-1)r * nCr = 28, then n is equal to
In the given question, an idiom/phrase has been underlined in the sentence. Choose the alternative which best expresses the meaning of the given idiom/phrase.
The police cordoned off the area after the explosion.
Read the passage carefully and answer the following question.
Nehru's was a many-sided personality. He enjoyed reading and writing books as much as he enjoyed fighting political and social evils or residing tyranny. In him, the scientist and the humanist were held in perfect balance. While he kept looking at special problems from a scientific standpoint. He never forgot that we should nourish the total man. As a scientist, he refused to believe in a benevolent power interested in men's affairs, but, as a self-proclaimed non-believer, he loved affirming his faith in life and the beauty of nature and the children he adored. Unlike Wordsworth, he did not see him trailing clouds of glory from the recent sojourn in heaven. He saw them as a blossom of promise and renewal, the only hope for mankind.
Which of the statements reflects Nehru's point of view?


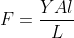
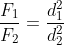
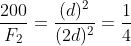





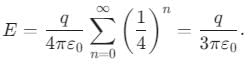

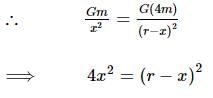
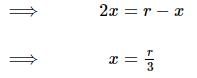
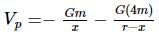
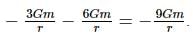
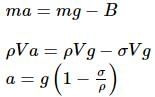
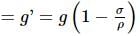
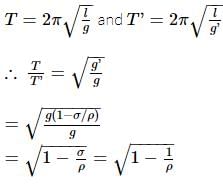
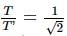
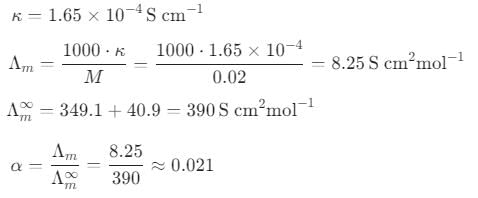
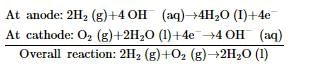
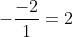
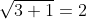
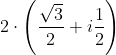
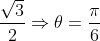
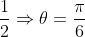
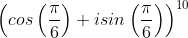
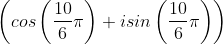
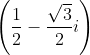
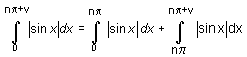
 dx = n
dx = n  dx = 2n
dx = 2n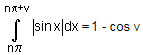
 = 2n + 1 - cos v
= 2n + 1 - cos v 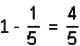
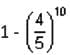
 equals
equals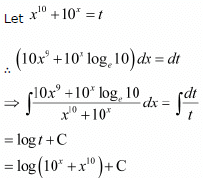
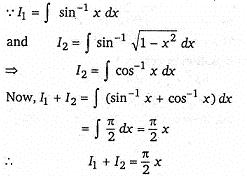

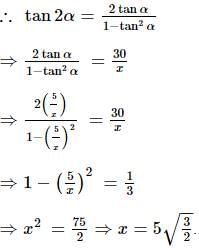
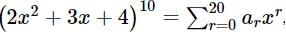 then the value of
then the value of 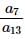 is
is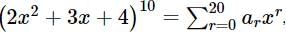 ...(1)
...(1)