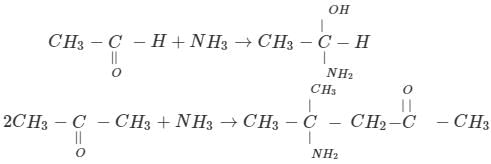SRMJEEE Chemistry Mock Test - 1 - JEE MCQ
30 Questions MCQ Test - SRMJEEE Chemistry Mock Test - 1
Acetaldehyde and acetone differ in their reaction with
Among the following hydrocarbons which decolourise bromine water, but does not give precipitate with ammonical AgNO3 is
A triglyceride can have how many different acyl groups?
The order of filling of electrons in the orbitals of an atom is
In a mole of water vapours at STP, the volume actually occupied or taken by the molecules (i.e., Avogadro's No.xvolume of one molecule) is
Pi-bonds do not alter the shape, but merely shorten the
Which of the following phenomenon will occur when two atoms of the elements having same spin of electron approach for bonding?
Chemical equilibrium is dynamic in nature, because
The bond dissociation energies of H₂,Cl₂ and HCl are 104, 58 and 103 kcal respectively. The enthalpy of formation of HCl gas will be
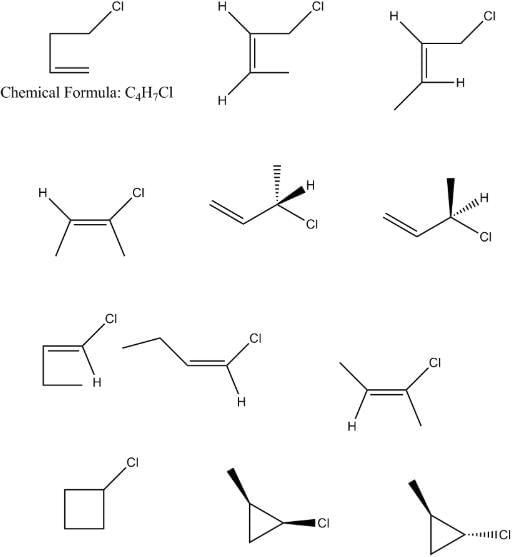
The total number of acyclic isomers including stereoisomers of C₄H₇Cl are
Which of the following ions are paramagnetic in character?
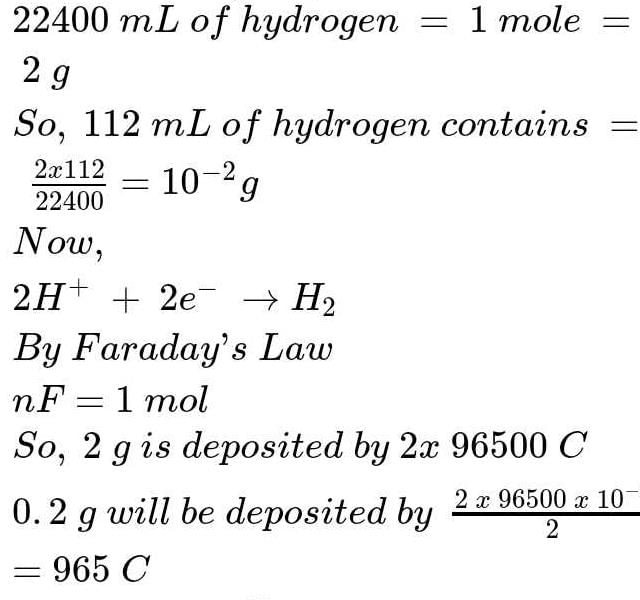
The charge required to liberate 112 ml of hydrogen from acidified water is
The value of gas constant per degree per mole is approximately
The alkene formed as a major product in the above elimination reaction is
When ammonium chloride is added to ammonia solution , the pH of the resulting solution will be
A compound with molecular formula C₇H₁₆ shows optical isomerism, the compound will be
Which of the following chemicals are used to manufacture methyl isocyanate that caused "Bhopal Tragedy " ? (a) Methyl amine (b) Phosgene (c) Phosphine (d) Dimethyl amine
The phenomenon of radioactivity was discovered by
If 0.183 of an aromatic monobasic acid requires 15ml of N/10 sodium hydroxide for complete neutralisation, then molecular weight of the acid is