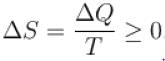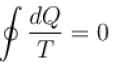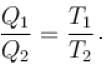Second Law Of Thermodynamic MCQ Level – 1 (Part - 2) - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Second Law Of Thermodynamic MCQ Level – 1 (Part - 2)
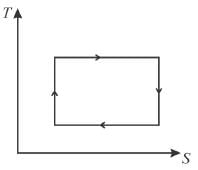
T-S diagram for Carnot cycle is.
Select one:
Select one:
Which of the following statement is/are correct regarding the Second Law of Thermodynamics?
1. No process is possible whose sole result is the absorption of heat from a reservoir and complete conversion of the heat into work.
2. No process is possible whose sole result is the transfer of heat from a colder object to a hotter object.
All natural processes are irreversible. This is a direct consequence of.
Select one:
Select one:
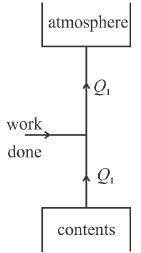
In a refrigerator, the heat exhausted to the outer atmosphere is.
Select one:
A given amount of heat cannot be completely converted into work. However, it is possible to convert a given amount of work completely in heat. This statement results from the
Select one:
The temperature of the surface of the sun is approximately 6000K. It we take a big lens and focus the sun rays and produce a temperature of 8000K. This will violate
Select one:
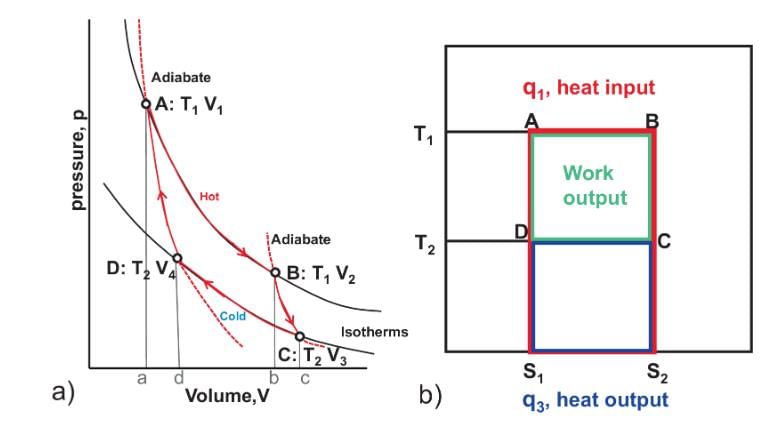
The area of the Carnot cycle on a T-S diagram represents
Select one:
For any process, the second law of thermodynamics requires that the change of entropy of universe is.
Select one:
For a reversible cyclic process, the value of 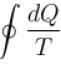 is.
is.
Select one:
In a heat engine maximum heat that can be converted into mechanical work.
Select one: