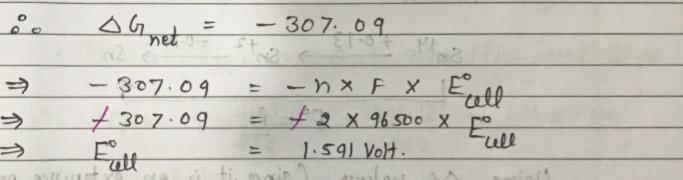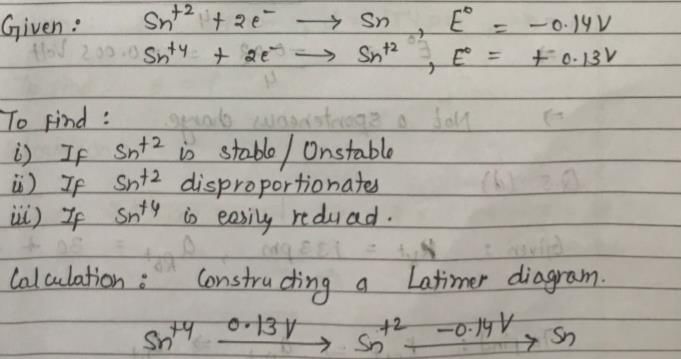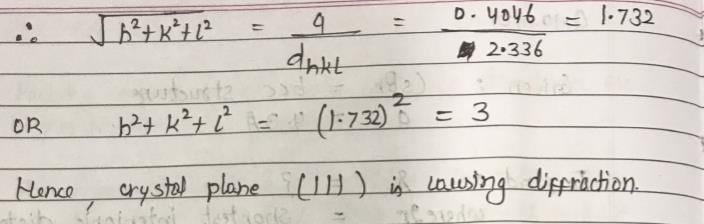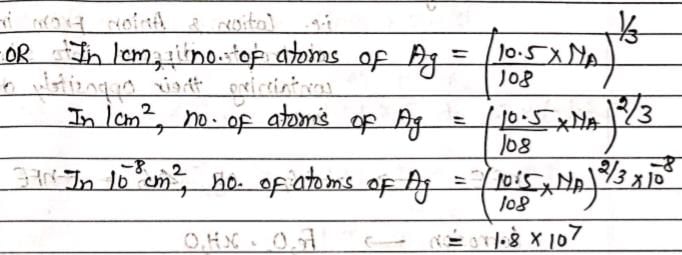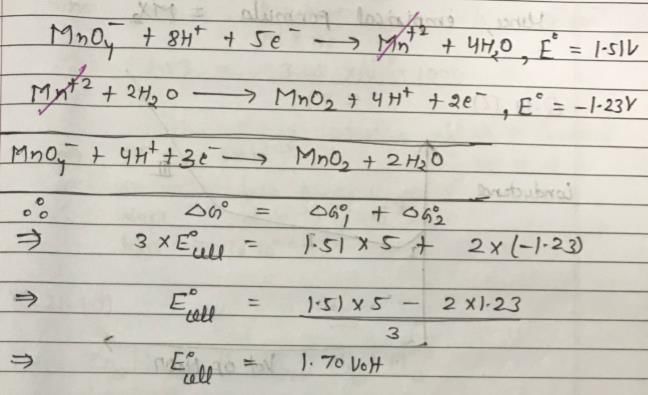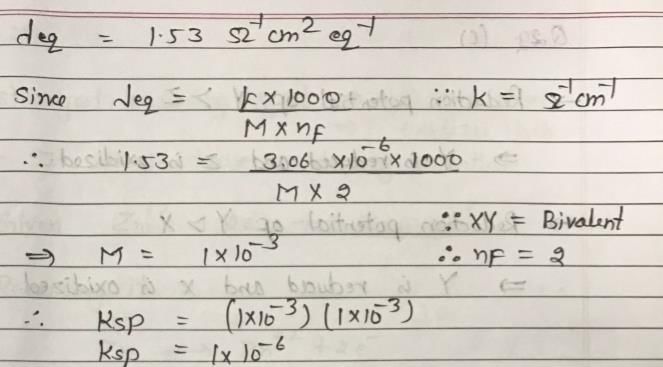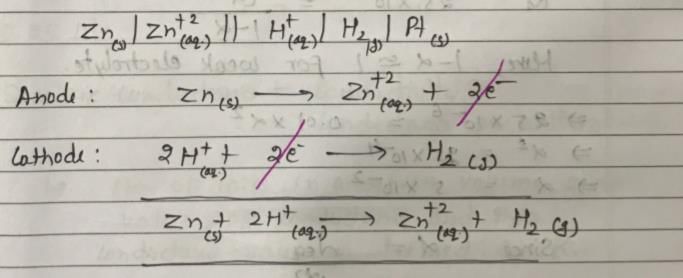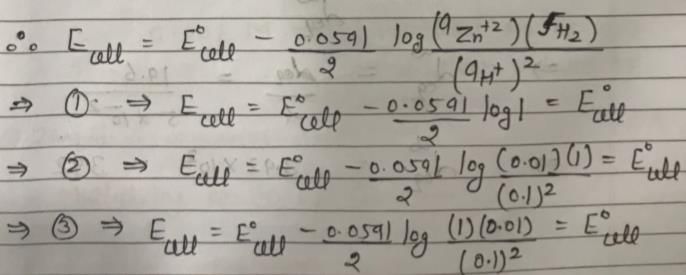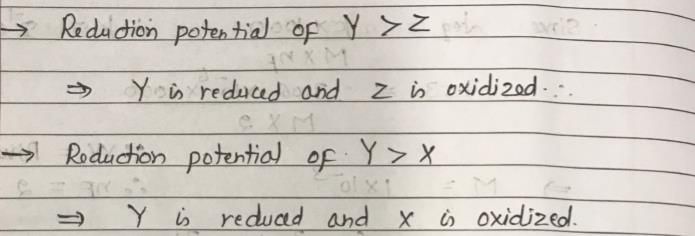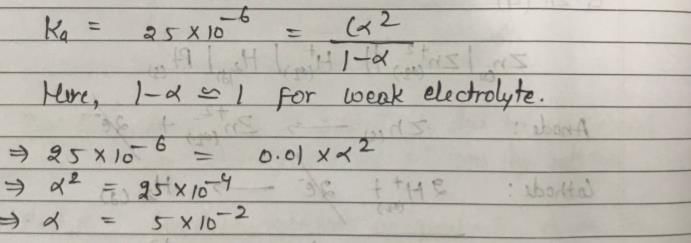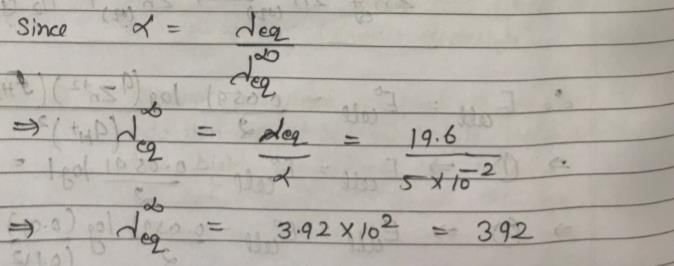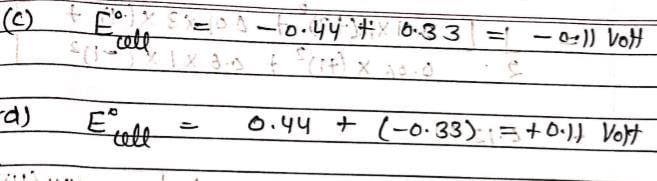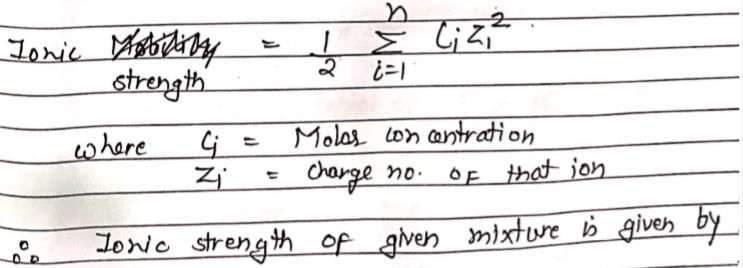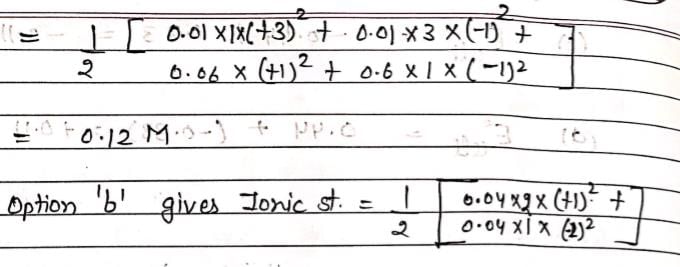Solid State- Electrochemistry- Conductance - Chemistry MCQ
30 Questions MCQ Test - Solid State- Electrochemistry- Conductance
Br– ion forms a closed packed structure. If the radius of it is 195 pm. Which of the following is true?
In fcc crystal lattice, edge length is 400 pm. Find the diameter of greatest sphere which can be fitted into the interstitial vo id without distortion of lattice:
For a Ag-Zn button cell, net reaction is, Zn(s) + Ag2O(s) → ZnO(s) + 2Ag(s) ΔGf(Ag2O) = -11.21 kJ mol-1, ΔGf(ZnO) = -318.3 kJ mol-1, 1 Faraday = 96500C Hence, E°cell of the button cell is:
Sn2+ + 2e– → Sn, E0 = – 0.14 V
Sn4+ + 2e- → Sn2+, E0 = + 0.13 V
Which of the following is true?
Each Rubidium halide crystallizing in the NaCl type lattice has a unit cell length 30 pm greater than for corresponding potassium salt (RK+ = 133 pm) of the same halogen. What is ionic radius of Rb+?
At what glancing angle would the first order diffraction occur, when copper radiation (wavelength = 154 pm) interacts with lattice planes that are 154 pm apart?
% of free space in ccp and bcc structure are, respectively:
In fcc lattice, atom A occupies the corner position and B occupies the face center positions. If one atom of B is missing from one of the Face centered positions, the formula of compound is?
The distance between two successive (110) planes in a simple cubic lattice with lattice parameter ‘a’ is:
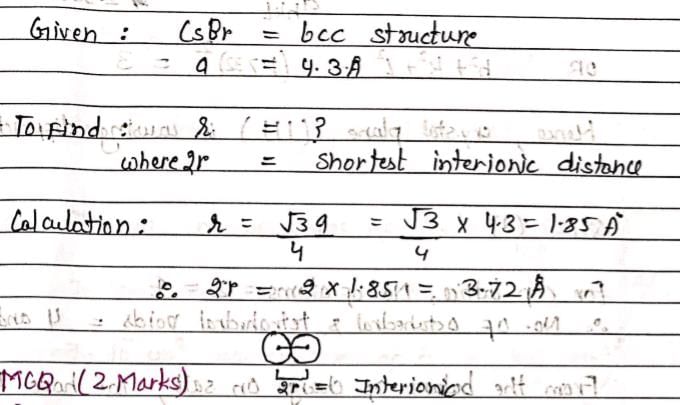
CsBr crystal has a bcc structure. It has an edge length of 4.3 Å. The shortest interionic distance between cation and anion is
Cu metal crystallizes with fcc structure. The density of crystal is 2698 kg/m3 and mass is 26.98 Kg. X-ray of wavelength 1.537 nm are diffracted by the crystal and the Bragg’s angle is 19.20. Identify the crystal plane which caused this diffraction. (NA = 6.023 × 1023)
If unit cell of a material has ccp array of Oxygen atoms with ‘m’ fraction of Octahedral holes occupied by Aluminium ions and ‘n’ fraction of Tetrahedral holes occupied by Magnesium ions. Then ‘m’ and ‘n’ are respectively:
Silver has a density of 10.5 g/cm3. The number of Silver atoms on a surface of area 10–12 m2 can be expressed in scientific notation as y × 10x. The value of ‘x’ is? (Given. At.Wt of silver = 108g/mol)
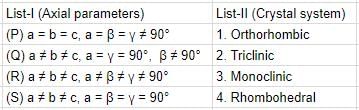
Match axial parameters given in List-I with crystal systems given in List-II and select the correct answer using the codes given below the lists:
Consider the following equations for a cell reaction:
A+B → C+D E0 = x; Keq = k1
2A+2B → 2C +2D E0 = y; Keq = k2
For the reactions,
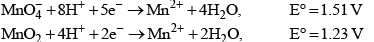
Then, for the reaction, 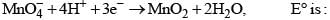
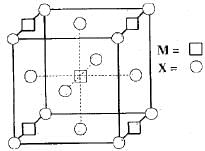
A compound MpXq has cubic close packing (ccp) arrangement of X. Its unit cell structure is shown below. The empirical formula of the compound is:
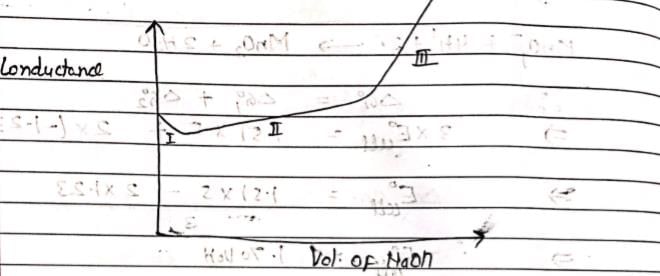
CH3COOH is titrated with NaOH solution. Which is true statement:
The conductivity of the saturated solution of some bivalent salt XY is 3.06 × 10–6 ohm–1 cm–1 and its equivalent conductivity is 1.53 ohm–1 cm2 equiv–1. The value of Ksp of XY is:
In the following electrochemical cell: Zn|Zn+2||H+|H2(Pt) and E0cell = Ecell
This is possible when:
The X at 1 atm is bubbled through a solution containing a mixture of 1 MY– and 1 MZ– at 25°C. If the order of reduction potential is Z < Y > X, then:
The ionization constant of a weak electrolyte is 25 × 10–6 while the equivalent conductance of its 0.01 M solution is 19.6 s cm2 eq–1. The equivalent conductance of the electrolyte at infinite dilution (in 5 cm2 eq–1) will be:
The standard reduction potential at 298 K for the following half cells are given:

Which is the strongest reducing agent:
Which of the following solutions of KCl has the lowest value of specific conductance?
Consider the given data:

E° for the reaction, 
For the electrochemical cell, 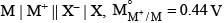
= V and M° X / X- = 0.33 V.
= From this data we can deduce that:
The specific conductivity of solution depends upon:
In a solution, cations H+, Li+, Na+ and K+ are present. The one with highest ionic mobility and another with lowest ionic mobility respectively are:
An aqueous solution containing 0.01 M FeCl3 and 0.06 M HClO4 has the same ionic strength as a solution of: