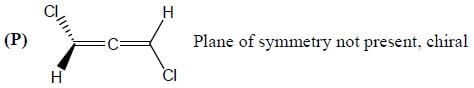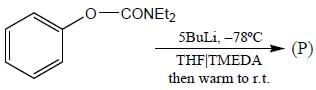GATE Chemistry Exam > GATE Chemistry Tests > GATE Chemistry Mock Test Series > Stereochemistry & Asymmetric Synthesis - GATE Chemistry MCQ
Stereochemistry & Asymmetric Synthesis - GATE Chemistry MCQ
Test Description
20 Questions MCQ Test GATE Chemistry Mock Test Series - Stereochemistry & Asymmetric Synthesis
Stereochemistry & Asymmetric Synthesis for GATE Chemistry 2025 is part of GATE Chemistry Mock Test Series preparation. The Stereochemistry & Asymmetric Synthesis questions and answers have been
prepared according to the GATE Chemistry exam syllabus.The Stereochemistry & Asymmetric Synthesis MCQs are made for GATE Chemistry 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Stereochemistry & Asymmetric Synthesis below.
Solutions of Stereochemistry & Asymmetric Synthesis questions in English are available as part of our GATE Chemistry Mock Test Series for GATE Chemistry & Stereochemistry & Asymmetric Synthesis solutions in
Hindi for GATE Chemistry Mock Test Series course. Download more important topics, notes, lectures and mock
test series for GATE Chemistry Exam by signing up for free. Attempt Stereochemistry & Asymmetric Synthesis | 20 questions in 60 minutes | Mock test for GATE Chemistry preparation | Free important questions MCQ to study GATE Chemistry Mock Test Series for GATE Chemistry Exam | Download free PDF with solutions
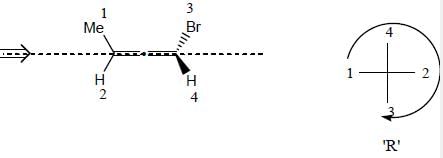
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 1
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 2
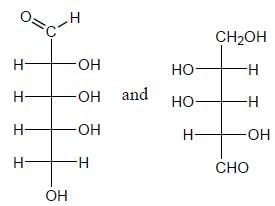
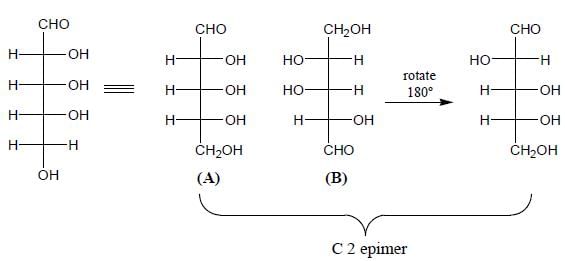
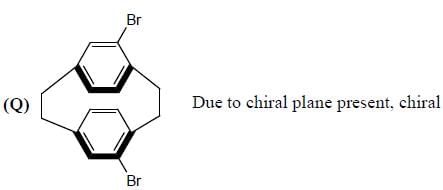
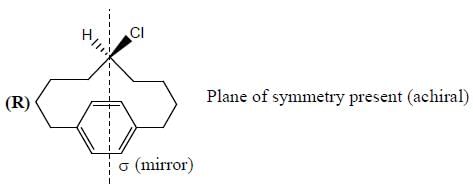
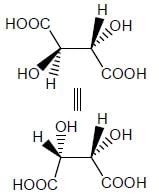
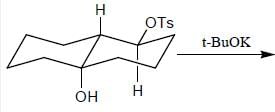
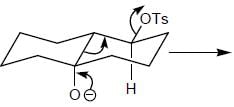
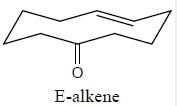
Stereochemistry & Asymmetric Synthesis - Question 3
Amongest the following the correct statement for the compound P, Q and R is
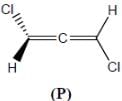
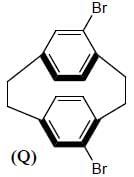
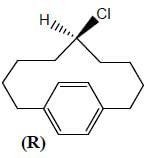



Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 3
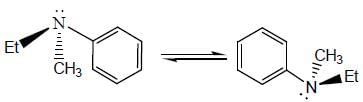
Stereochemistry & Asymmetric Synthesis - Question 4
Among the following, the optically inactive compounds is/are
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 4
*Answer can only contain numeric values
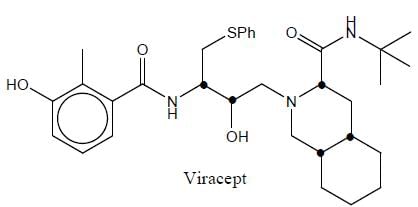
Stereochemistry & Asymmetric Synthesis - Question 5
Vivacept used in the treatment of HIN and mestranol is an oral contraceptive. The total number of asymmetric centre in viracept are
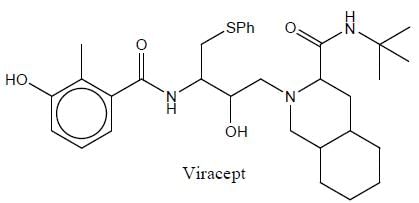
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 5
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 6
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 7
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 8
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 9
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 10
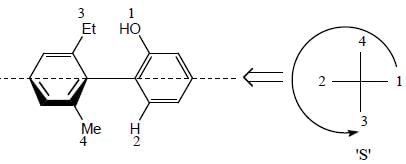
Stereochemistry & Asymmetric Synthesis - Question 11
The absolute configurations for compounds X and Y respectively are
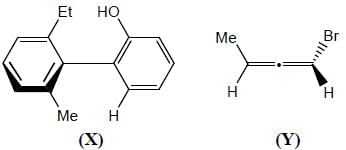
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 11
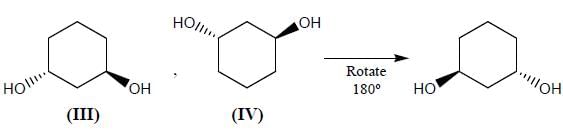
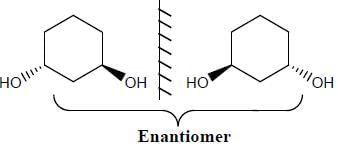
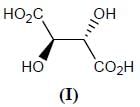
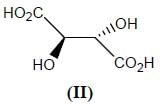
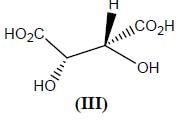
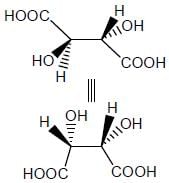
Stereochemistry & Asymmetric Synthesis - Question 12
Which two of the following compounds represents a pair of enantiomers.
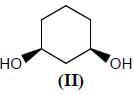
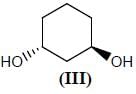
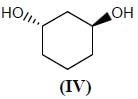
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 12
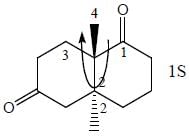
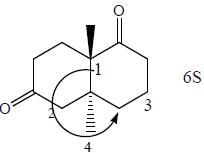
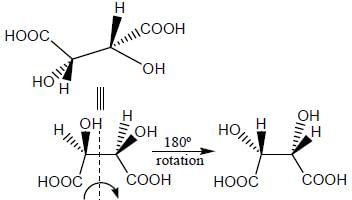
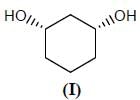
Stereochemistry & Asymmetric Synthesis - Question 13
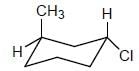
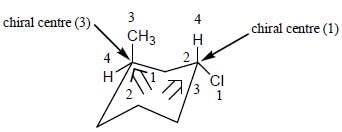
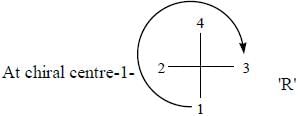
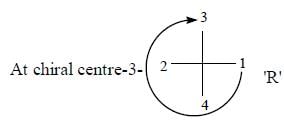
The configuration (R/S notation) at C-1 and C-6 of the compound below are
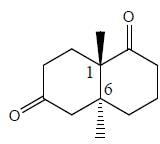
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 13
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 14
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 15
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 16
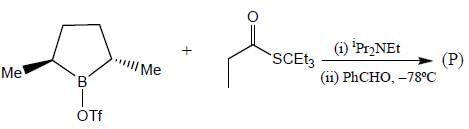
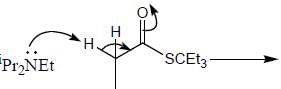
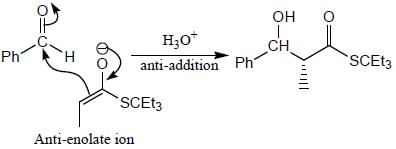
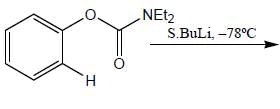
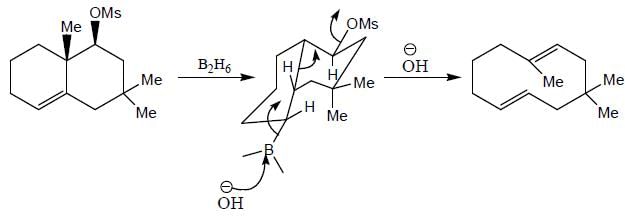
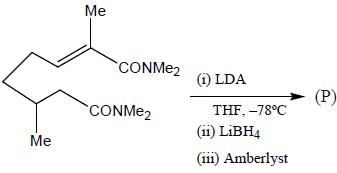
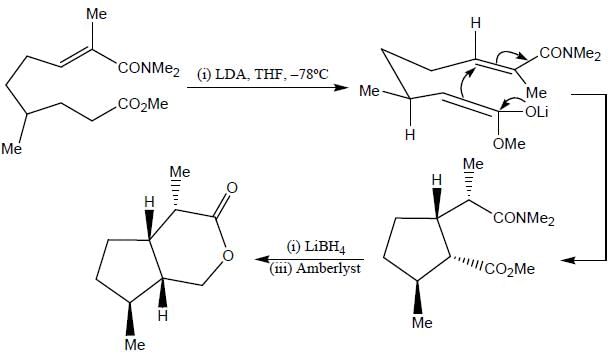
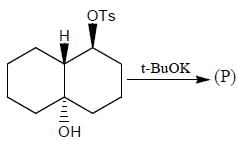
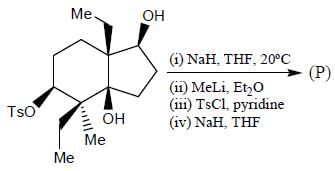
Stereochemistry & Asymmetric Synthesis - Question 17
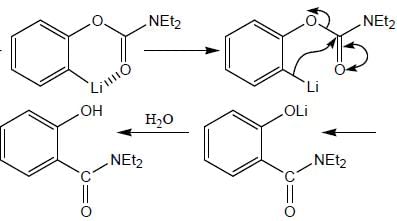
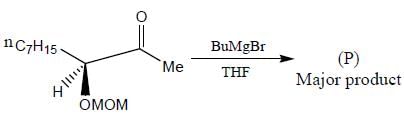
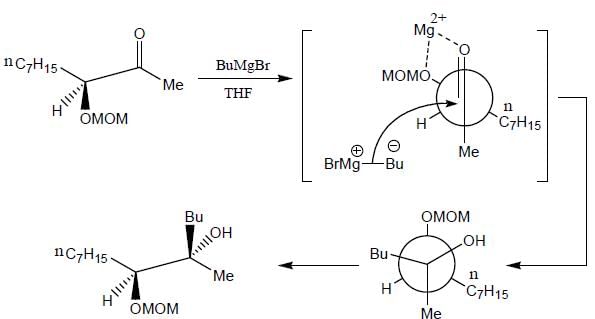
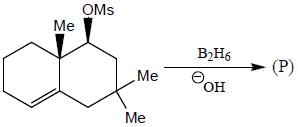
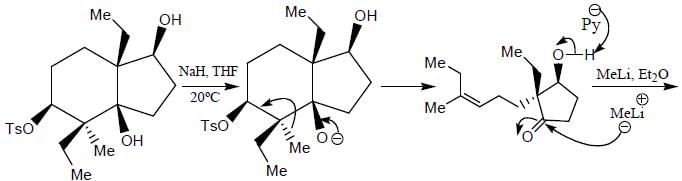
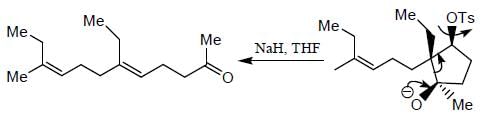
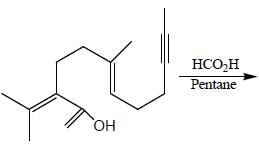
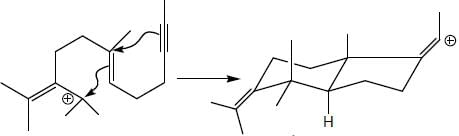
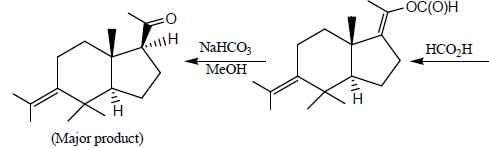
The major product formed in the following reaction sequence
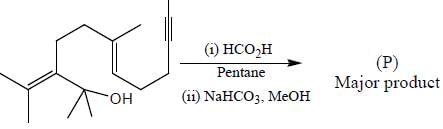
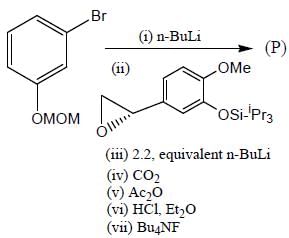
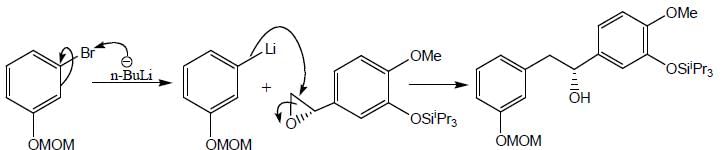
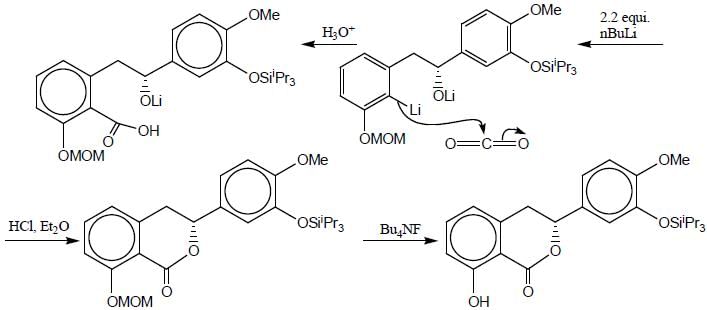
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 17
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 18
*Answer can only contain numeric values
Stereochemistry & Asymmetric Synthesis - Question 19
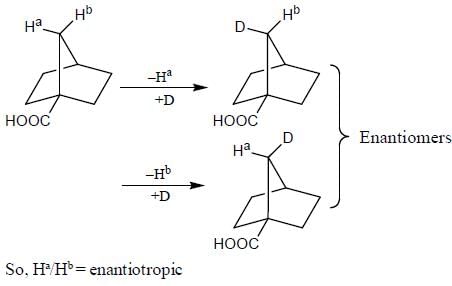
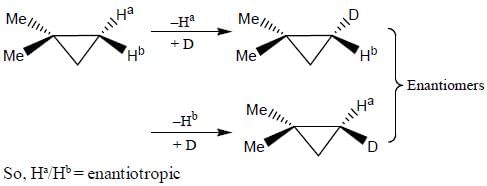
In the following how many structures (Hydrogens marked Ha and Hb) are enantiotopic.
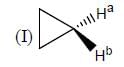
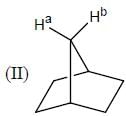
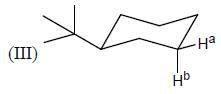
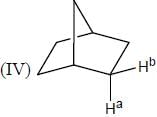
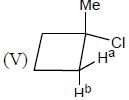
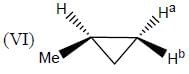
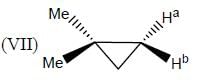
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 19
*Answer can only contain numeric values
Stereochemistry & Asymmetric Synthesis - Question 20
How many in the following compounds is/are chiral
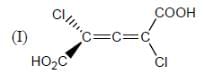
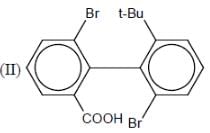
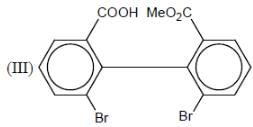
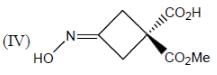
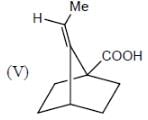
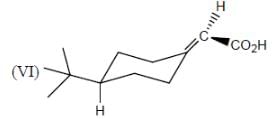
Detailed Solution for Stereochemistry & Asymmetric Synthesis - Question 20
|
20 docs|37 tests
|
Information about Stereochemistry & Asymmetric Synthesis Page
In this test you can find the Exam questions for Stereochemistry & Asymmetric Synthesis solved & explained in the simplest way possible.
Besides giving Questions and answers for Stereochemistry & Asymmetric Synthesis, EduRev gives you an ample number of Online tests for practice