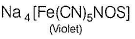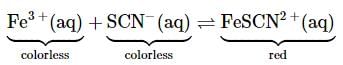Test: Bonding in Organometallic Compounds, Stability & Applications of Coordination Compounds - JEE MCQ
25 Questions MCQ Test - Test: Bonding in Organometallic Compounds, Stability & Applications of Coordination Compounds
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q. A filter paper moistened with ammoniacal sodium nitroprusside solution turns violet on contact with a drop of alkaline Na2S solution .The violet colour is due to the formation of
Wilkinson’s catalyst is
Tollen’s reagent contains
The compound formed by dissolving gold and platinum in aqua-regia is
Among the following, which is not the n-bonded organometallic compound?
Which of the following biomolecules contain a non-transition metal ion?
In black and white photography, the developed film is fixed by washing with hypo solution which dissolves the undecomposed AgBr to form a complex ion formed is
In the estimation of hardness of water, the reagent used is
Which of the following is powerful cr-donor and n-acceptor ligand?
Fe2+ and Fe3+ can be distinguished by
Orie or More than One Options Correct Type
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Which of the following forms a metal carbonyl corresponding [M(CO)6]?
In which of the following porphyrin acts as ligand?
Which are π-bonded organometallic compounds?
Which is the correct?
How many of the following combinations in an aqueous medium will give a blue colour or precipitate?
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Coordination compounds play a vital role in our lives. The importance of these compounds can be realised from the fact that life would not have been possible without the existence of chlorophyll in plants and haemoglobin in the blood of the animals. The field of such compounds has expanded very fast in recent years and coordination compounds are playing important roles in analytical chemistry, polymerisation reactions, metallurgy and refining of metals, etc.
Q.
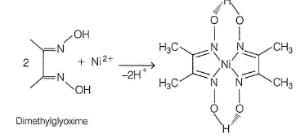
The reagent used for identifying nickel ion is
Coordination compounds play a vital role in our lives. The importance of these compounds can be realised from the fact that life would not have been possible without the existence of chlorophyll in plants and haemoglobin in the blood of the animals. The field of such compounds has expanded very fast in recent years and coordination compounds are playing important roles in analytical chemistry, polymerisation reactions, metallurgy and refining of metals, etc.
Q.
Which is used in cancer chemotherapy?
Matching List Type
Direction (Q. Nos. 18 and 19) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d) out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
Match the Column I with Column II and mark the correct option from the codes given below.
One Integer Value Correct Type
Direction (Q. Nos. 20-24) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
The number of terminal carbonyl groups present in Fe2(CO)9 are
In Fe2(CO)9, the number of CO present between iron atoms ......
In [Co2(CO)8], the number of CO molecules lying between the metal atoms are
The number of M—M bonds in [Ir4(CO)12] are
In Mn2(CO)10, the number of CO molecules in between the metal atoms are
Statement Type
Direction (Q. No. 25) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Mn(π— C3H5)(CO)4, obey effective atomic number rule.
Statement II : π-allyl ligand act as a 3e- donor.