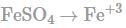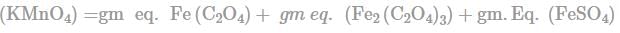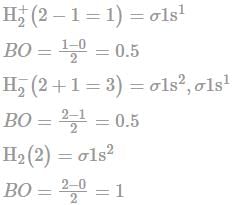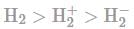Test: CSIR-NET Chemical Sciences Mock Test - 5 - UGC NET MCQ
30 Questions MCQ Test - Test: CSIR-NET Chemical Sciences Mock Test - 5
If log 27 = 1.431, then the value of log 9 is
Three number are in the ratio of 3 : 4 : 5 and their L.C.M. is 2400. Their H.C.F. is:
Two dice are tossed. The probability that the total score is a prime number is:
If selling price is doubled, the profit triples. Find the profit percent ?
If the diagonal of a rectangle is 17cm long and its perimeter is 46 cm. Find the area of the rectangle.
An accurate clock shows 8 o'clock in the morning. Through how may degrees will the hour hand rotate when the clock shows 2 o'clock in the afternoon?
In a certain code language COMPUTER is written as RFUVQNPC. How will MEDICINE be written in that code language?
Two bikes are moving with speeds m km/h and n km/h towards a crossing along two perpendicular roads. If their distances from the crossing be 60 meters and 70 meters respectively at an instant of time then they do not collide if their speeds are such that
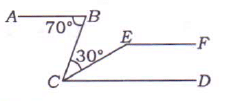
In the given figure, if AB || CD || EF, then the measure of ∠CEF is
The sum of any three consecutive natural numbers where first number is even number is always divisible by?
A clock takes 5 seconds to strike 5 times at 5 O'clock. How long will it take to strike 9 times at 9 O'clock ?
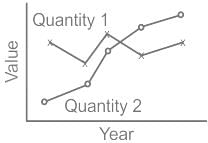
The trends of two quantities over five years are shown in the graph. Which of the following are valid inferences?
A. The mean values of the quantities are nearly equal
B. The variations in the two quantities are nearly equal
C. Quantity 1 varies less over the given period as compared to Quantity 2
The bar chart given below shows the total revenue (in Rs. '000) earned by 5 shops by selling steel bottles.
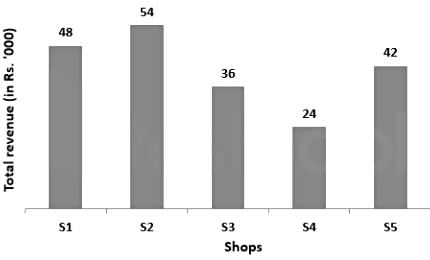
The price of each bottle is Rs. 600 in all the shops. How many bottles have been sold by S2 and S3 taken together?
Study the following graph and answer the question that follows.
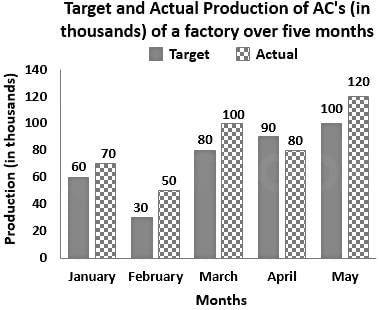
The actual production of ACs in March is what percentage more than the average target production of ACs over the period of 5 months (correct to the nearest integer)?
At what angle are the hands of a clock at 15 minutes past 4 o'clock?
Consider the following reactions and choose an option correctly indicating the chemical property of water:
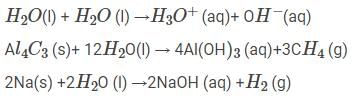
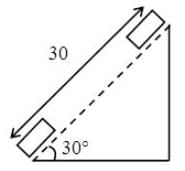
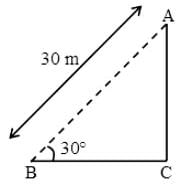
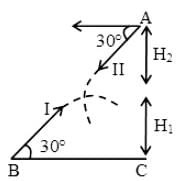
Two guns are pointed at each other one upwards at an angle of elevation of 30º and other at the same angle of depression, the muzzle being 30 m apart. If the charges leave the gun with velocities of 350 m/s and 300 m/s respectively. Find when will they meet?
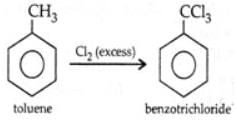
By passing excess Cl2(g) in boiling toluene, which one of the following compounds is exclusively formed?
Which of the following compounds is not coloured ?
Iodoform can be prepared from all, except
Which one of the following represents the correct ratio of the energy of electron in ground state of H atom to that of the electron in the first excited state of Li+?
The bivalent metal ion having maximum paramagnetic behavior among the first transition series elements is,
Which of the following is correct representation of bleaching powder?
What is the pH of a saturated solution of Mg(OH)2? Ksp = 1.8 x 10-11.
Electrons accelerated from rest by a potential difference of 12.75 V, are bombarded on a monoatomic hydrogen gas. Possible emission of spectral lines are -
In order to oxidise a mixture of one mole of each of FeC2O4,Fe(C2O4)3,FeSO4 and Fe2(SO4)3 in acidic medium, the number of moles of KMnO4 required is:
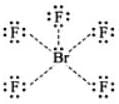
CO is a stronger ligand that Cl- because
The variation of solubility of four different gases (G1, G2, etc.) in a given solvent with pressure at a constant temperature is shown in the plot.
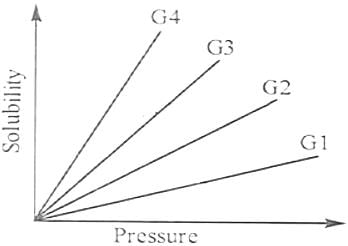
The gas with the highest value of Henry’s Law constant is
The bond length of H2+,H2-, and H2 are in the following order



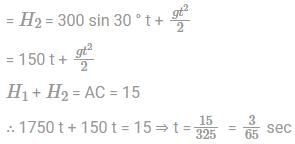
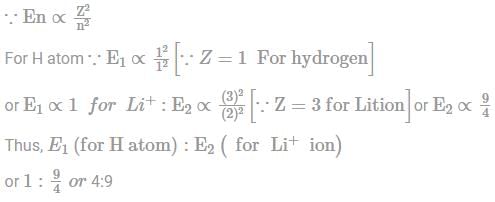
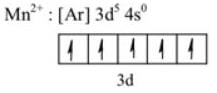
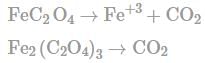
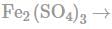 No oxidation because all elements are already in highest oxidation stake
No oxidation because all elements are already in highest oxidation stake