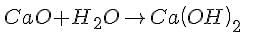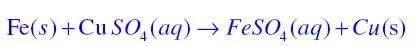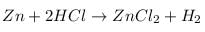Test: Chemical Reactions & Equations - 4 - RRB Group D / RPF Constable MCQ
25 Questions MCQ Test General Science for Competitive Exams - Test: Chemical Reactions & Equations - 4
Which of the following product is formed when calcium oxide reacts with water?
The reactions in which more reactive element can displace less reactive element from a compound are called
Decomposition of ferrous sulphate into Fe2O3,SO2 and SO3 occurs in the presence of
Find the incorrect statement :
(I) oxygen is highly combustible and hydrogen is supporter of combustion,
(II) Oxygen and hydrogen both are highly combustible,
(III) Oxygen and hydrogen both are supporters of combustion,
(IV) Hydrogen is highly combustible and oxygen is supporter of combustion
Which of the following decolourise a blue solution of copper sulphate?
Which one of Fe, Al, Cu, Zn is the least reactive metal?
The volume of gas collected in one of the test tubes in electrolysis of water is double than other name the gas
Milk becomes sour if kept for a long time because of
What happens when dilute HCl is added to iron fillings?
Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
What happens when copper metal is added to silver nitrate solution?
Fe2O3+2Al→Al2O3+2Fe This reaction is an example of -
Solutions of zinc sulphate (I), copper sulphate (II), aluminum sulphate (III) and iron sulphate (IV)
were taken in four test tubes. An iron nail was put in each solution and the system was left undisturbed for an hour. In which set up or set ups the iron nail be coated?
A student added zinc granules to copper sulphate solution taken in a test tube. Out of the following, the correct observation (s) made by the student will be
I. zinc granules have no regular shape.
II. Zinc granules have silvery grey colour.
III. The colour of zinc granules changed to brownish black.
When a student added zinc granules to dilute HCl, a colour less and odourless gas was evolved, which was tested with a burning match stick it was observed that
If the solution of lead nitrate and potassium iodide are mixed together. What is the colour of the precipitate obtained?
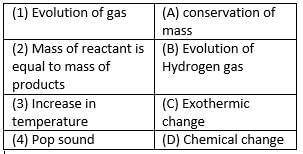
Match the following with correct response.
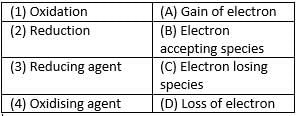
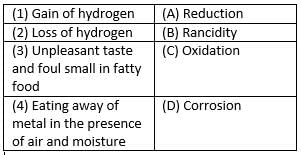
Match the following with the correct response.
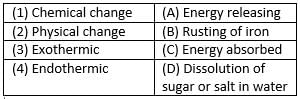
Match the following with correct response.
Match the following with correct response.
|
365 docs|169 tests
|