Test: Chemistry - 3 - MCAT MCQ
15 Questions MCQ Test MCAT Mock Test Series 2025 - Test: Chemistry - 3
The IUPAC notation for representing an atom is 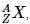 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:
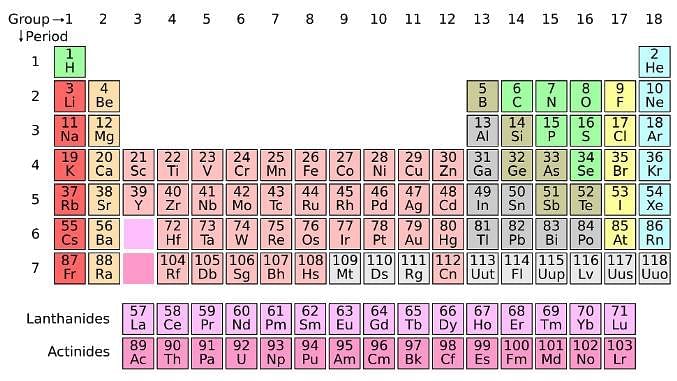
The location of an element in the periodic table influences its properties. Among the most important periodic trends are ionization energy, which increases from left to right and from bottom to top; atomic radius, which increases from right to left and from top to bottom; and electron affinity, which increases from left to right and from bottom to top.
Q. Which of the following pairs of elements has the same number of electrons on its valence layer? (See attachment.)
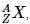 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:

The IUPAC notation for representing an atom is 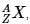 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:
The location of an element in the periodic table influences its properties. Among the most important periodic trends are ionization energy, which increases from left to right and from bottom to top; atomic radius, which increases from right to left and from top to bottom; and electron affinity, which increases from left to right and from bottom to top.
Q. Which of the following elements has the smallest ionization energy? (See attachment.)
 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:

The IUPAC notation for representing an atom is 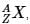 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:
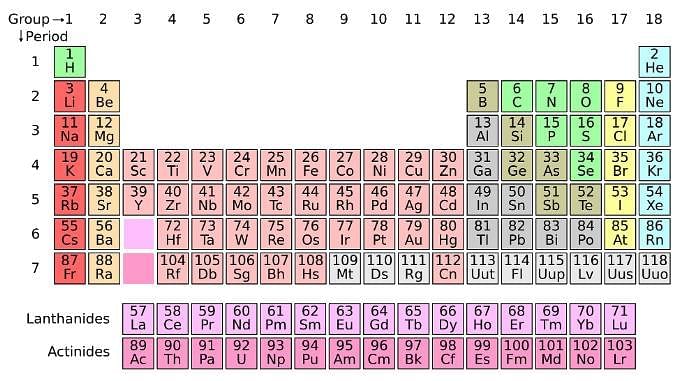
The location of an element in the periodic table influences its properties. Among the most important periodic trends are ionization energy, which increases from left to right and from bottom to top; atomic radius, which increases from right to left and from top to bottom; and electron affinity, which increases from left to right and from bottom to top.
Q. A scientist is investigating the electric attraction between charged ions in salts. The research finds that the electric force is inversely proportional to the size of the charged ions. Which of the following salts would have the highest force of attraction? (See attachment.)
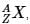 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:

The IUPAC notation for representing an atom is 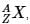 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:
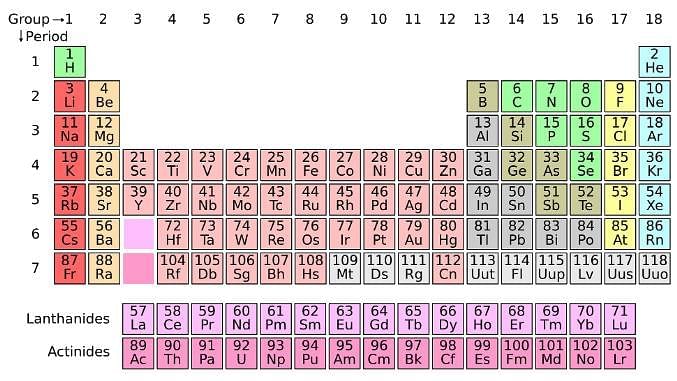
The location of an element in the periodic table influences its properties. Among the most important periodic trends are ionization energy, which increases from left to right and from bottom to top; atomic radius, which increases from right to left and from top to bottom; and electron affinity, which increases from left to right and from bottom to top.
Q. Which of the following pairs of atoms has the same number of neutrons? (See attachment.)
The IUPAC notation for representing an atom is 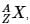 where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
where A is the mass number (number of protons + neutrons), Z is the atomic number (number of protons), and X is the element’s chemical symbol. The Z number is often omitted and the number of protons is usually found from the element’s symbol, as all atoms of the same element have the same atomic number.
The periodic table serves as a way of arranging elements based on their increasing atomic numbers. On the periodic table, elements with the same number of electrons in their valence layer are located in the same group (vertical row), and elements with the same principal energy level of their valence layer are located in the same period (horizontal row). The periodic table is shown in the image below:
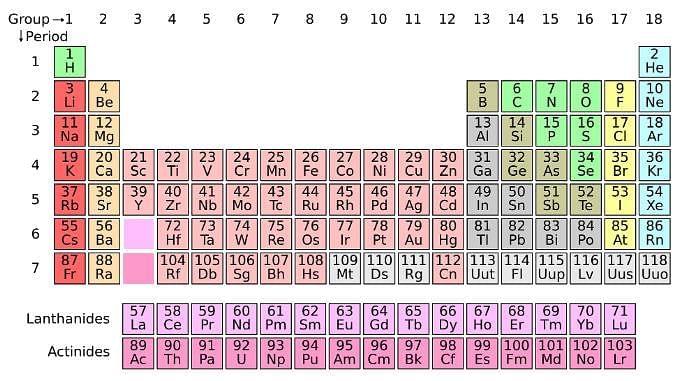
The location of an element in the periodic table influences its properties. Among the most important periodic trends are ionization energy, which increases from left to right and from bottom to top; atomic radius, which increases from right to left and from top to bottom; and electron affinity, which increases from left to right and from bottom to top.
Q. Which of the following elements is most likely to form a negative ion? (See attachment.)
A chemical solution is a homogeneous mixture of one or more substances called “solutes” in another substance called “solvent”. The concentration of a solution is the amount of solute that is dissolved in a certain amount or solvent or solution.
There are different units to represent the concentration of a solution, among them there are the molarity (M), defined as the moles of solute present in 1 L of solution; molality, (m) defined as the moles of solute dissolved in 1 kg of solvent; and percentage by mass (%m), which represents the grams of solute dissolved in 100 g of solution.
Q. What mass of KBr is needed to prepare 250 mL of a 0.200 M solution of KBr?MM KBr = 119.002 g/mol (See attachment.)
A chemical solution is a homogeneous mixture of one or more substances called “solutes” in another substance called “solvent”. The concentration of a solution is the amount of solute that is dissolved in a certain amount or solvent or solution.
There are different units to represent the concentration of a solution, among them there are the molarity (M), defined as the moles of solute present in 1 L of solution; molality, (m) defined as the moles of solute dissolved in 1 kg of solvent; and percentage by mass (%m), which represents the grams of solute dissolved in 100 g of solution.
Q. A bottle of concentrated hydrochloric acid has a mass percent of 37.2% and a density of 1.18 g/mL. What is the molarity of concentrated hydrochloric acid? (You may consult the attachment.)
A chemical solution is a homogeneous mixture of one or more substances called “solutes” in another substance called “solvent”. The concentration of a solution is the amount of solute that is dissolved in a certain amount or solvent or solution.
There are different units to represent the concentration of a solution, among them there are the molarity (M), defined as the moles of solute present in 1 L of solution; molality, (m) defined as the moles of solute dissolved in 1 kg of solvent; and percentage by mass (%m), which represents the grams of solute dissolved in 100 g of solution.
Q. If 3.20 g of NaNO3 are dissolved in enough water to make 0.500 L of solution, what is the molality of the solution? (Assume the density of the solution is 1.00 g/mL). MM NaNO3 = 84.9947 g/mol (See attachment.)
Nucleophilic substitution reactions are organic reactions in which an electron-rich substance called a nucleophile reacts with an organic substrate that has an electron-poor carbon atom called an electrophile.
The electrophile has a radical that is a weak base and is substituted by the nucleophile called the leaving group, hence the term nucleophilic substitution.
Nucleophilic substitution can occur via two different mechanisms: SN1, in which the rate determining step is the loss of the leaving group, and SN2, in which the rate-determining step is the nucleophilic attack and the simultaneous loss of the leaving group.
A scientist is studying the nucleophilic substitution reaction between 2-Bromopropane and a novel nucleophile (Nuc), collecting the kinetic data that is shown in the table below:
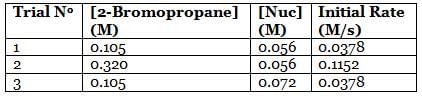
Q. Referring to the attachment, which of the following is the strongest nucleophile?
Nucleophilic substitution reactions are organic reactions in which an electron-rich substance called a nucleophile reacts with an organic substrate that has an electron-poor carbon atom called an electrophile.
The electrophile has a radical that is a weak base and is substituted by the nucleophile called the leaving group, hence the term nucleophilic substitution.
Nucleophilic substitution can occur via two different mechanisms: SN1, in which the rate determining step is the loss of the leaving group, and SN2, in which the rate-determining step is the nucleophilic attack and the simultaneous loss of the leaving group.
A scientist is studying the nucleophilic substitution reaction between 2-Bromopropane and a novel nucleophile (Nuc), collecting the kinetic data that is shown in the table below:
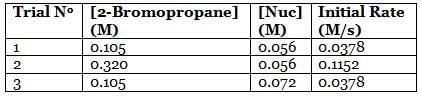
Q. Using the attachment, what is the rate constant for the nucleophilic substitution reaction of 2-Bromopropane with the novel nucleophile Nuc?
Nucleophilic substitution reactions are organic reactions in which an electron-rich substance called a nucleophile reacts with an organic substrate that has an electron-poor carbon atom called an electrophile.
The electrophile has a radical that is a weak base and is substituted by the nucleophile called the leaving group, hence the term nucleophilic substitution.
Nucleophilic substitution can occur via two different mechanisms: SN1, in which the rate determining step is the loss of the leaving group, and SN2, in which the rate-determining step is the nucleophilic attack and the simultaneous loss of the leaving group.
A scientist is studying the nucleophilic substitution reaction between 2-Bromopropane and a novel nucleophile (Nuc), collecting the kinetic data that is shown in the table below:
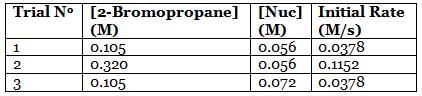
Q. The reaction between 2-Bromopropane and the novel nucleophile Nuc proceeds via an ____ mechanism in which the rate law is ____. (You may consult the attachment.)
Which of the following amino acids will be located in the inside of a folded protein?
Which of the following lipids is not found in the cell membrane?
Which of the following aqueous solutions would have the lowest freezing point?
|
45 tests
|



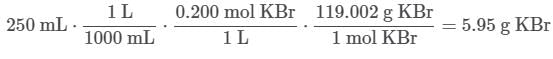
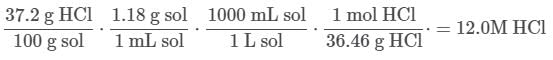

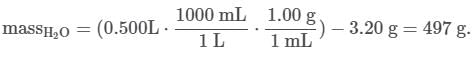
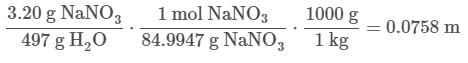
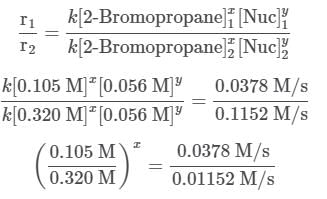
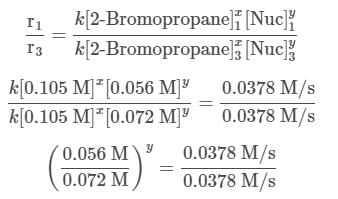
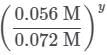 needs to be equal to 1, it means that y = 0.
needs to be equal to 1, it means that y = 0.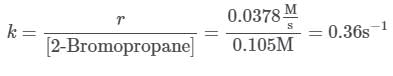
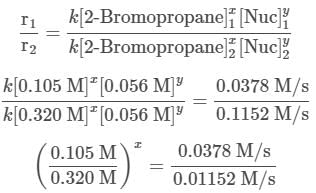
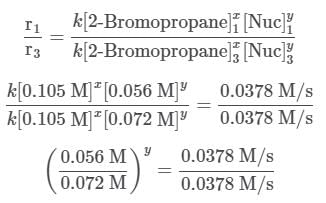
 needs to be equal to 1, it means that y = 0. These reaction orders show that the reaction proceeds through an SN1 mechanism in which the rate law is r = k⋅[2 − Bromopropane]
needs to be equal to 1, it means that y = 0. These reaction orders show that the reaction proceeds through an SN1 mechanism in which the rate law is r = k⋅[2 − Bromopropane]










