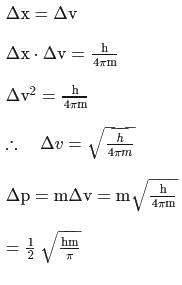Test: Dual Behaviour of Matter, Heisenberg Uncertainty Principle - JEE MCQ
15 Questions MCQ Test - Test: Dual Behaviour of Matter, Heisenberg Uncertainty Principle
If a suitable photon is employed to locate an electron (mass = 9.11 × 10−31 kg) in an atom within a distance of 10.98 nm, the uncertainty involved in the measurement of its velocity in ms−1 is
The position of both, an electron and a helium atom is known within 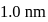 . Further the momentum of the electron is known within
. Further the momentum of the electron is known within 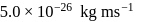 . The minimum uncertainty in the measurement of the momentum of the helium atom is
. The minimum uncertainty in the measurement of the momentum of the helium atom is
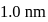 . Further the momentum of the electron is known within
. Further the momentum of the electron is known within 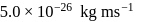 . The minimum uncertainty in the measurement of the momentum of the helium atom is
. The minimum uncertainty in the measurement of the momentum of the helium atom isAt temperature T, the average kinetic energy of any particle is 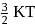 . The de Broglie wavelength follows the order:
. The de Broglie wavelength follows the order:
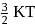 . The de Broglie wavelength follows the order:
. The de Broglie wavelength follows the order:The de-Broglie wavelength of a tennis ball of mass 60 g moving with a velocity of 10 ms−1 is approximately ____ (Planck's constant h = 6.63 × 10−34 J.s)
If the de-Broglie wavelength of a particle of mass m is 100 times its velocity, then its value in terms of its mass (m) and Planck's constant (h) is
Calculate the velocity of ejected electron from the metal surface when light of frequency 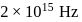 fall on the metal surface and the threshold frequency is
fall on the metal surface and the threshold frequency is 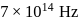 for metal ?
for metal ?
Two fast moving particles 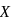 and
and 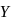 are associated with de Broglie wavelengths
are associated with de Broglie wavelengths 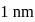 and
and  respectively. If mass of
respectively. If mass of 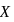 is nine times the mass of
is nine times the mass of 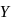 , the ratio of kinetic energies of
, the ratio of kinetic energies of 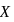 and
and 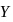 would be
would be
The energy required to remove an electron from the surface of Sodium metal is 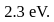 What is the longest wavelength of radiation with which it can show photoelectric effect?
What is the longest wavelength of radiation with which it can show photoelectric effect?
The velocity of particle 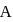 is
is 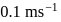 and that of particle
and that of particle  is
is 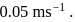 If the mass of particle
If the mass of particle 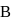 is five times that of particle
is five times that of particle 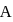 , then the ratio of deBroglie wavelengths associated with the particles
, then the ratio of deBroglie wavelengths associated with the particles 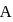 and
and 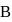 is
is
A light of frequency 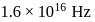 when falls on a metal plate emits electrons that have double the kinetic energy compared to the kinetic energy of emitted electrons when frequency of
when falls on a metal plate emits electrons that have double the kinetic energy compared to the kinetic energy of emitted electrons when frequency of  falls on the same plate. The threshold frequency
falls on the same plate. The threshold frequency 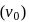 of the metal in
of the metal in 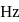 is
is
The work function (ϕ) of some metals is listed below. The number of metals which will show photoelectric effect when light of 300 nm wavelength falls on the metals is:
If uncertainty in position and velocity are equal then uncertainty in momentum will be


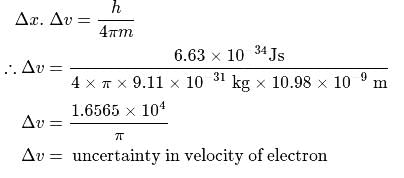
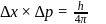 (which is constant).
(which is constant).
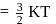
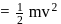
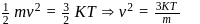
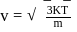

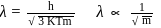

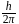 i.e.,
i.e., 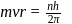
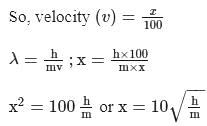
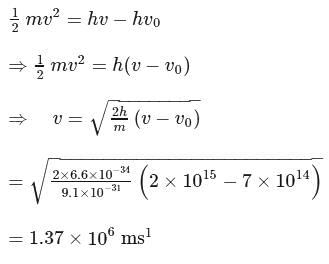
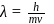

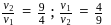
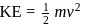
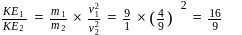
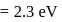
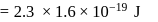
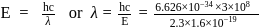
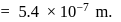
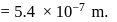
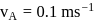 and
and 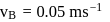 also,
also,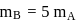
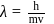
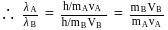
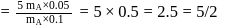
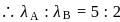
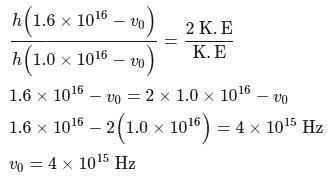
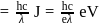
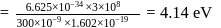
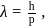 there is inverse relation between de Broglie wavelength and momentum, hence the graph will be rectangular hyperbola.
there is inverse relation between de Broglie wavelength and momentum, hence the graph will be rectangular hyperbola.