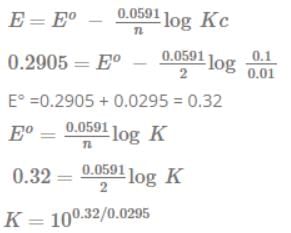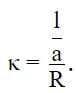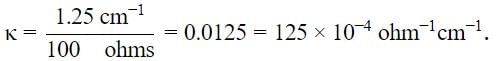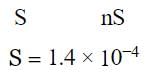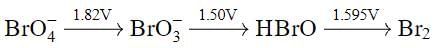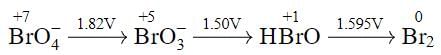Test: Electrochemistry - 1 - NEET MCQ
20 Questions MCQ Test - Test: Electrochemistry - 1
The standard reduction potential at 298 K for the following half cells are given:
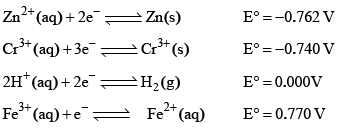
Which is the strongest reducing agent:
Which of the following conditions are satisfied when the cell reaction in the electrochemical cell is spontaneous?
What is the observation when the opposing external applied potential to an electrochemical cell is greater than the cell’s potential?
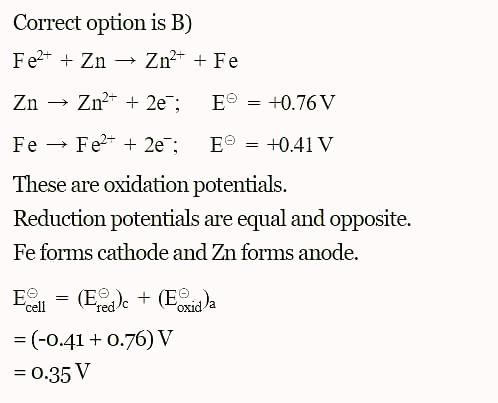
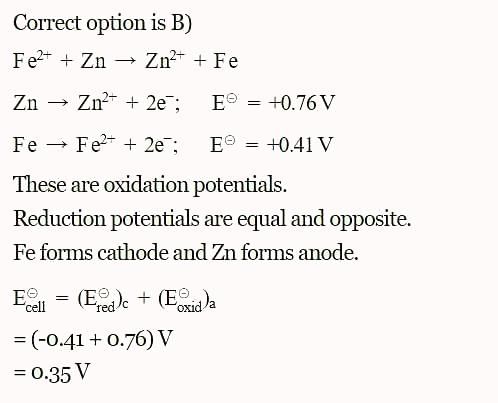
The standard reduction potentials E°, for the half reactions are as:
The emf for the cell reaction,
The correct order of equivalent conductance at infinite dilution of LiCl, NaCl and KCl is:
Saturated solution of KNO3 is used to make ‘salt-bridge’ because:
The specific conductances of four electrolytes in ohm−1cm−1 are given below. Which one offers higher resistance to passage of electric current?
The emf of the above cell is 0.2905 V. Equilibrium constant for the cell reaction is:
Given, standard electrode potentials, Fe2+ + 2e- →Fe, E° = -0.440V
Fe3+ +3e- → Fe, E° = -0.036V
The standard electrode potential (E°) for Fe3+ + e- → Fe2+ is:
Based on the data given blow strongest oxidizing agent will be:
The measured resistance of a conductance cell was 100 Ω. (MKCl = 74.5 gmol−1 and cell constant =1.25 cm−1). If the specific conductance in ohm−1 cm−1 is 125 × 10−x, then what is the value of x?
The solubility of a certain sparingly soluble substance MXn is nearly 1.4 × 10−4 M. If the solubility product is 1.1 × 10−11, what is the value of n?
The electrochemical cell shown below is a concentration cell.
M|M2+ (saturated solut ion of a sparingly so luble salt, MX2 || M2+ (0.001 mol dm–3)| M.
The emf of the cell depends on the difference in concentration of M2+ ions at the two electrodes. The emf of the cell at 298 is 0.059 V.
Q.
The value of ΔG (kJ mol-1) for the given cell is (take 1 F = 96500 C mol-1)
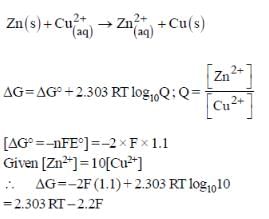
For the following cell,
Zn(s) | ZnSO4(aq) || CuSO4(aq) | Cu(s)
When the concentration of Zn2+ is 10 times the concentration of Cu2+, the expression for ΔG (in J mol-1) is [F is Faraday constant; R is gas constant; T is temperature; E° (cell) = 1.1 V]
An electrochemical cell consists of two half-cell reactions.
The mass of copper (in grams) dissolved on passing 0.5 A current for 1h is [Given, atomic mass of Cu is 63.6, F = 96500 C mol–1]
Using the following Latimer diagram for bromine, pH = 0;
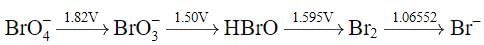
the species undergoing disproportionation is:
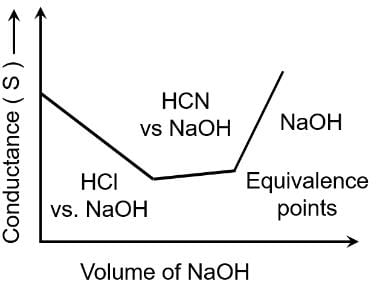
Conductometric titration curve of an equimolar mixture of HCl and HCN with NaOH (aq) can be given as
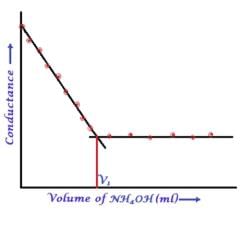
In a typical Conductometric titration of a strong acid with a weak base, the curve resembles: