Test: Gas Phase - MCAT MCQ
10 Questions MCQ Test - Test: Gas Phase
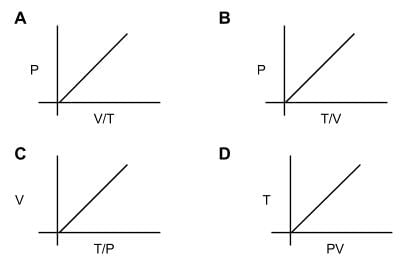
How many diagrams showing relationships between pressure, volume, and temperature below is/are incorrect for ideal gases?

At a given temperature T and pressure P, a person's lung holds a set volume of V of air. Given that air is roughly 20% oxygen, how many moles of oxygen are in his lungs?
Gases found in the environment are most likely to exhibit properties similar to that of ideal gases under conditions of:
Assume an experiment vessel which is well fitted with a piston. The airtight piston has negligible mass and can move up and down freely. There is 1 mol of an ideal gas contained within the vessel under the piston at pressure of 100 kPa. The piston rests at 10 cm from the base of the vessel. The pressure in the vessel increases to 200 kPa as force is applied to the piston. What would be the new resting position of the piston from the base of the vessel? Assume the temperature is held constant at 300 K throughout the experiment.
Which properties reflected in real gases does the van der Waals equation attempt to account for by modifying the ideal gas law?
I. Volume
II. Pressure
III. Temperature
The behavior of which of these real gases will be reflected most closely with the ideal gas law?
Assuming ideal gas properties, which of the following occupies the most volume at 273 K and 1 atm of pressure?
Atmospheric air is comprised, roughly, of 80% nitrogen and 20% oxygen. A 100 L sample of atmospheric air is kept at 300 K and 100 kPa. How many moles of oxygen molecules are found in this gas sample? (Use R = 10)
(L⋅kPa)/(mol⋅K))
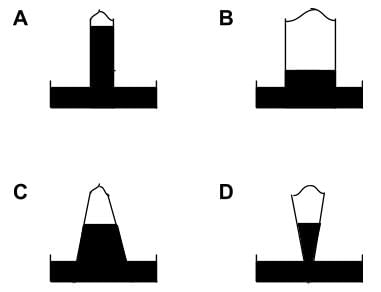
Shown below are four mercury barometers of the same height (all four barometer tubes measure one meter from the tube opening to rounded top). Which barometer shows the greatest external pressure?