Class 10 Exam > Class 10 Tests > Science Class 10 > Test: Important Chemical Compounds - 1 - Class 10 MCQ
Test: Important Chemical Compounds - 1 - Class 10 MCQ
Test Description
15 Questions MCQ Test Science Class 10 - Test: Important Chemical Compounds - 1
Test: Important Chemical Compounds - 1 for Class 10 2024 is part of Science Class 10 preparation. The Test: Important Chemical Compounds - 1 questions and answers have been
prepared according to the Class 10 exam syllabus.The Test: Important Chemical Compounds - 1 MCQs are made for Class 10 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Important Chemical Compounds - 1 below.
Solutions of Test: Important Chemical Compounds - 1 questions in English are available as part of our Science Class 10 for Class 10 & Test: Important Chemical Compounds - 1 solutions in
Hindi for Science Class 10 course. Download more important topics, notes, lectures and mock
test series for Class 10 Exam by signing up for free. Attempt Test: Important Chemical Compounds - 1 | 15 questions in 10 minutes | Mock test for Class 10 preparation | Free important questions MCQ to study Science Class 10 for Class 10 Exam | Download free PDF with solutions
Test: Important Chemical Compounds - 1 - Question 1
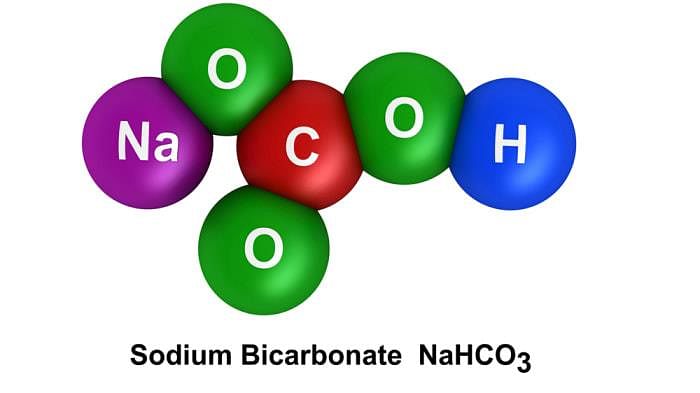
What is the chemical formula for baking soda?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 1
Test: Important Chemical Compounds - 1 - Question 2
How is washing soda produced from baking soda?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Important Chemical Compounds - 1 - Question 3
What is the primary use of baking soda in managing stomach-related issues?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 3
Test: Important Chemical Compounds - 1 - Question 4
Which industry commonly uses washing soda as a cleaning agent?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 4
Test: Important Chemical Compounds - 1 - Question 5
What is the process in which a solid substance is formed, with atoms or molecules arranged in a strong structure called a crystal?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 5
Test: Important Chemical Compounds - 1 - Question 6
Which of the following materials is NOT mentioned as an example of a substance with a crystalline structure?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 6
Test: Important Chemical Compounds - 1 - Question 7
What is the term used to describe the water that combines with a salt to form crystals?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 7
Test: Important Chemical Compounds - 1 - Question 8
Which process involves the direct formation of a solid substance from a gas without going through a liquid phase?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 8
Test: Important Chemical Compounds - 1 - Question 9
What is the chemical formula for Plaster of Paris?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 9
Test: Important Chemical Compounds - 1 - Question 10
For what purpose is Plaster of Paris commonly used in orthopedic medicine?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 10
Test: Important Chemical Compounds - 1 - Question 11
What makes Plaster of Paris a popular material in sculpting and art?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 11
Test: Important Chemical Compounds - 1 - Question 12
In addition to sculpting and art, where else is Plaster of Paris commonly used?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 12
Test: Important Chemical Compounds - 1 - Question 13
What is the chemical formula of bleaching powder?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 13
Test: Important Chemical Compounds - 1 - Question 14
What happens at the cathode during the electrolysis of brine?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 14
Test: Important Chemical Compounds - 1 - Question 15
What is the primary use of bleaching powder in the textile industry?
Detailed Solution for Test: Important Chemical Compounds - 1 - Question 15
|
85 videos|437 docs|75 tests
|
Information about Test: Important Chemical Compounds - 1 Page
In this test you can find the Exam questions for Test: Important Chemical Compounds - 1 solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Important Chemical Compounds - 1, EduRev gives you an ample number of Online tests for practice