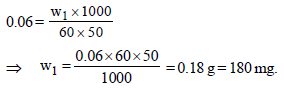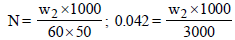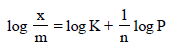Test: JEE Main 35 Year PYQs- The Solid State & Surface Chemistry - JEE MCQ
26 Questions MCQ Test - Test: JEE Main 35 Year PYQs- The Solid State & Surface Chemistry
The formation of gas at the surface of tungsten due toadsorption is the reaction of order [2002]
Na and Mg crystallize in BCC and FCC type crystalsrespectively, then the number of atoms of Na and Mg presentin the unit cell of their respective crystal is [2002]
How many unit cells are present in a cube-shaped idealcrystal of NaCl of mass 1.00 g ? [2003]
[Atomic masses : Na = 23, Cl = 35.5]
[Atomic masses : Na = 23, Cl = 35.5]
Which one of the following characteristics is not correct forphysical adsorption ? [2003]
What type of crystal defect is indicated in the diagram below?

Identify the correct statement regarding enzymes [2004]
An ionic compound has a unit cell consisting of A ions atthe corners of a cube and B ions on the centres of the facesof the cube. The empirical formula for this compound wouldbe [2005]
The volume of a colloidal particle, VC as compared to the volume of a solute particle in a true solution VS , could be
The disperse phase in colloidal iron (III) hydroxide andcolloidal gold is positively and negatively charged,respectively. Which of the following statements is NOTcorrect ? [2005]
Total volume of atoms present in a face-centred cubic unit cell of a metal is (r is atomic radius) [2006]
In Langmuir's model of adsorption of a gas on a solid surface[2006]
In a compound, atoms of element Y form ccp lattice andthose of element X occupy 2/3rd of tetrahedral voids. Theformula of the compound will be [2008]
Gold numbers of protective colloids A, B, C and D are 0.50,0.01, 0.10 amd 0.005, respectively. the correct order of theirprotective powers is [2008]
Which of the following statements is incorrect regardingphysissorptions? [2009]
Copper crystallises in fcc with a unit cell length of 361 pm.What is the radius of copper atom? [2009]
The edge length of a face centered cubic cell of an ionicsubstance is 508 pm. If the radius of the cation is 110 pm, theradius of the anion is [2010]
Percentages of free space in cubic close packed structureand in body centered packed structure are respectively[2010]
In a face centred cubic lattice, atom A occupies the cornerpositions and atom B occupies the face centre positions. Ifone atom of B is missing from one of the face centred points,the formula of the compound is : [2011]
Lithium forms body centred cubic structure. The length ofthe side of its unit cell is 351 pm. Atomic radius of the lithiumwill be : [2012]
According to Freundlich adsorption isotherm which of the following is correct? [2012]
Which of the following exists as covalent crystals in thesolid state ? [JEE M 2013]
The coagulating power of electrolytes having ions Na+, Al3+and Ba2+ for arsenic sulphide sol increases in the order : [JEE M 2013]
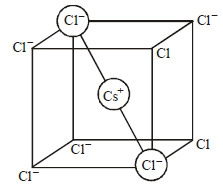
CsCl crystallises in body centred cubic lattice. If ‘a’ is its edge length then which of the following expressions is
correct?
Sodium metal crystallizes in a body centred cubic lattice witha unit cell edge of 4.29Å. The radius of sodium atom isapproximately : [JEE M 2015]
3 g of activated charcoal was added to 50 mL of acetic acidsolution (0.06N) in a flask. After an hour it was filtered andthe strength of the filtrate was found to be 0.042 N. Theamount of acetic acid adsorbed (per gram of charcoal) is : [JEE M 2015]
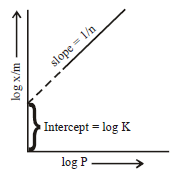
For a linear plot of log (x/m) versus log p in a Freundlichadsorption isotherm, which of the following statements iscorrect? (k and n are constants) [JEE M 2016]


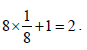
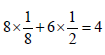

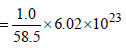
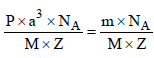
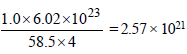 unit cells.
unit cells.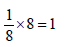
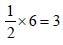
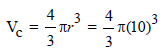
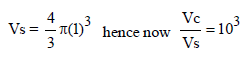

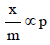
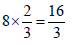
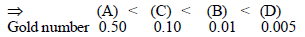
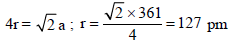
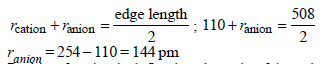
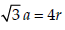
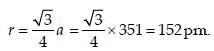
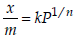
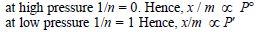
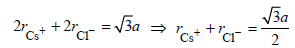
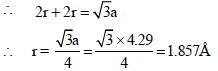
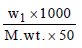 (Normality = 0.06 N)
(Normality = 0.06 N)