Test: Measurement of pH - GATE MCQ
20 Questions MCQ Test - Test: Measurement of pH
The hydrogen ion content in water goes from 0.203 g/L to 0.0032 g/L. How much does the pH change?
Which of the following is the value of hydrogen ion concentration of pure water?
Which of the following is the relation between hydrogen and hydroxyl ion concentration of pure water?
The Nernst equation is given by which of the following statements?
Which of the following is the relation between the concentration of hydrogen and hydroxyl ions in an acidic solution?
Which of the following is the relation between the concentration of hydrogen and hydroxyl ions in a basic solution?
The measurement of hydrogen ion concentration can be made by measuring the potential developed in an electrochemical cell.
In which of the following ways can zero drift be reduced in pH meters?
Which of the following can be used to provide automatic temperature compensation?
Which of the following is not the characteristic of null-detector type pH meter?
Which of the following is not the characteristic of direct-reading type pH meters?
Which of the following is not the characteristic of the chopper amplifier pH meter?
In which of the following ways can the disadvantages of chopper amplifier type pH meter be overcome?
The zero stability of vibrating condenser amplifier type pH meter is much better than a direct-coupled amplifier.
In vibrating condenser amplifier type pH meter, to maintain good performance which of the following has to be done?
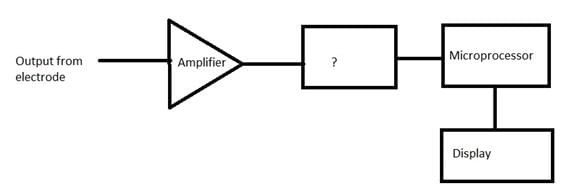
Given below is the block diagram of the digital pH meter. Identify the unmarked component.