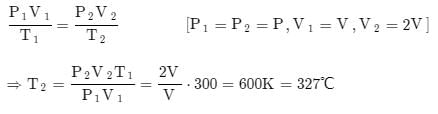Test: Thermodynamics Level - 2 - Mechanical Engineering MCQ
25 Questions MCQ Test - Test: Thermodynamics Level - 2
If a gas is heated against a pressure, keeping the volume constant, then work done will be equal to
Properties of substances like pressure, temperature and density, in thermodynamic coordinates are
Which of the following quantities is not the property of the system
According to avogadro's law, for a given pressure and temperature, each molecule of a gas
On weight basis, air contains following parts of oxygen
Which of the following is not the intensive property
Which of the following items is not a path function
Work done in an adiabatic process between a given pair of end state depends on
Which of the following parameters is constant for a mole for most of the gases at a given temperature and pressure
Which of the following quantities do not represent the property of the system.
If the value of n is high in the polytropic process, then the compressor work between given pressure limits will be
A perfect gas at 27°C is heated at constant pressure till its volume is double. The final temperature is
One volume basis, air contains following parts of oxygen
The molecular weight expressed in gm (i.e., 1 gm mole) of all gases at N.T.P. occupies a volume of
The specific heat of air increases with increase in
When a gas flows through a very long pipe of uniform cross section, the flow is approximately
The more effective way of increasing efficiency of Carnot engine is to
An expansion process as per law pV = constant is known as
When a liquid boils at constant pressure, the following parameter increases