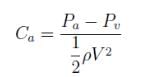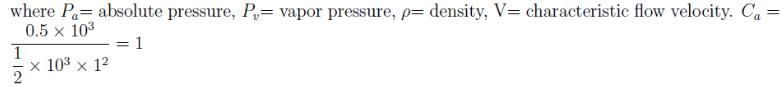Test: Vapor Pressure - Mechanical Engineering MCQ
8 Questions MCQ Test Fluid Mechanics for Mechanical Engineering - Test: Vapor Pressure
Which of the following statement is true about vapor pressure of a liquid?
Which of the following option correctly depicts the relation between the vapor pressure of a liquid and it’s temperature?
Which of the following is the condition for the boiling of a liquid?
Which of the following machines have the possibility of cavitation?
The three liquids 1, 2, and 3 with vapor pressures V1, V2 and V3 respectively, are kept under same pressure. If V1 > V2 > V3, which liquid will start boiling early?
Equal amount of a particular liquid is poured into three similar containers, namely 1, 2 and 3, at a temperature of T1, T2 and T3 respectively. If T1 < T2 < T3, the liquid in which container will have the highest vapor pressure?
The absolute pressure of a water is 0.5kN above it’s vapor pressure. If it flows with a velocity of 1m/s, what will be the value of Cavitation Number describing the flow induced boiling?
Which of the following is correct regarding the formation and collapse of vapor bubbles in a liquid?
|
56 videos|106 docs|75 tests
|