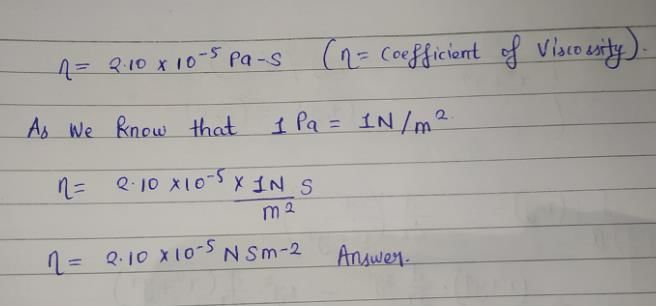Test: Viscosity & Surface Tension (Old NCERT) - JEE MCQ
20 Questions MCQ Test - Test: Viscosity & Surface Tension (Old NCERT)
Which of the following properties of water can be used to explain the spherical shape of rain droplets?
How does the surface tension of a liquid vary with increase in temperature?
Arrange the following in increasing order of surface tension.
I (water), II (ethanol), III (hexane)
Some of the following properties of liquids arise because of the molecular interaction and thermal energy.
I. Vapour pressure II. Surface tension III. Viscosity
Select the properties.
One of the following properties increase with increase in temperature,
Surface tension of water is 73 dynes cm -1 at 20° C. If surface area is increased by 0.10 m2, work done is
The coefficient of viscosity of a species is 2.10x 10-5 Pa-s. This value is also equal to
Direction (Q. Nos. 9) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q. Match the physical properties given in Column I with the corresponding units given in Column II and select the correct answer from the codes given below.
Direction (Q. Nos. 10-12) This section contains 3 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Select correct statement(s).
The temperature dependence of the surface tension of a liquid is given by the Sugden equation, .
Select correct statement(s).
Direction (Q. Nos. 13 and 18) This sectionis based on statement I and Statement II. Select the correct answer from the code given below
Q.
Statement I : Increase in temperature decreases viscosity of liquid.
Statement II : Increase in temperature and thus kinetic energy can overcome intermolecular forces of attraction.
Statement I : The order of viscosities o f hexane, water and glycerol is hexane < water < glycerol.
Statement II : Hexane has weakest intermolecular forces and glycerol the 1 strongest.
Statement I : Liquids tend to have maximum number of molecules at their surface.
Statement II : Small liquid drops have spherical shape.
Statement I : As temperature is increased, less work is required to extend the surface of a liquid therefore surface tension decreases.
Statement II : As the temperature and hence the intensity of molecular motion increases, intermolecular forces become less effective.
Statement I : Capillary action is due to surface tension.
Statement II : Mercury, with its strong cohesive forces and weaker adhesive forces, does not show capillary rise.
Statement I : A certain amount of energy called activation energy (Ea) is required to move into a hole.
Statement II : On increasing temperature, Ea is available and liquid can flow more easily thus, viscosity decreases.
Consider the following statements.
1. The viscosity of a gas increases with rise in temperature.
2. The viscosity of the liquid falls very rapidly with rise in temperature.
Which of the statements(s) given above is/are correct?
Variation of viscosity (q) with temperature T is given by
Q. Energy of activation required to move into a hole is