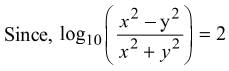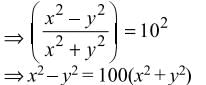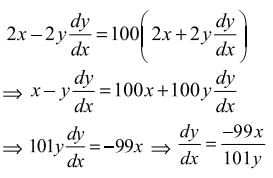UGEE SUPR Mock Test- 3 - JEE MCQ
30 Questions MCQ Test - UGEE SUPR Mock Test- 3
Two thin lenses are in contact and the focal length of the combination is 80 cm. If the focal length of one lens is 20 cm, then the power of the other lens will be
If the kinetic energy of a free electron doubles, it's de-Broglie wavelength changes by the factor
The ratio of the energies of the hydrogen atom in its first to second excited states is
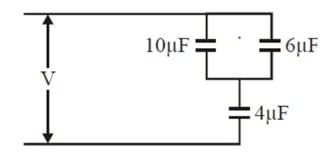
The equivalent capacitance of the combination of the capacitors is
The magnetic field at a distance r from a long wire carrying current i is 0.4 tesla. The magnetic field at a distance 2r is
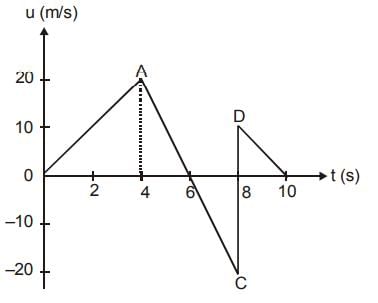
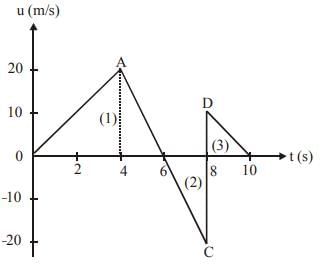
The velocity-time graph of a body moving in a straight line is shown in fig. Find the displacement and distance travelled by the body in 10 seconds.
An electric dipole is kept in a uniform electric field. It experiences
If tan θ = √n for some non-square natural number n, then sec2θ is:
If z = x + iy, z1/3 = a - ib, then 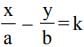 (a2 - b2) where k is equal to
(a2 - b2) where k is equal to
A circle has radius 3, and its centre lies on the line y = x - 1. The equation of the circle, if it passes through (7, 3), is
Derivative of log (sec θ + tan θ) with respect to sec θ at θ = π/4 is
The joint equation of bisectors of angles between lines x = 5 and y = 3 is
The point on the curve 6y = x3 +2 at which y - co-ordinate is changing 8 times as fast as x- co-ordinate is ______________
If the function f(x) defined by
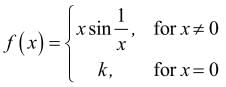
is contitnous at x = 0, then k is equal to
If ‘n’ represents the total number of asymmetric carbon atoms in a compound, then the possible number of optical isomers of the compound is
How many Faradays of electricity are required to deposit 10 g of calcium from molten calcium chloride using inert electrodes?
(Molar mass of calcium = 40 g mol-1)
The compound which is not formed when a mixture of n-butyl bromide and ethyl bromide treated with sodium metal in the presence of dry ether is
The amine ‘A’ when treated with nitrous acid gives yellow oily substance. The amine A is


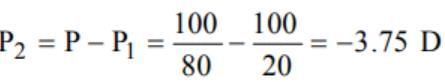
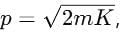 where m is the mass and K is the kinetic energy.
where m is the mass and K is the kinetic energy.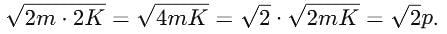
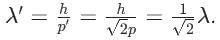

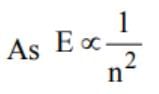
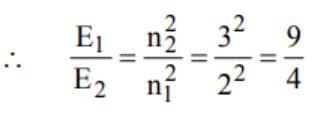
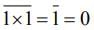
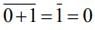
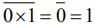
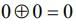
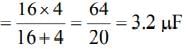
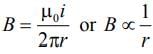
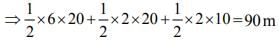
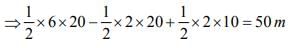
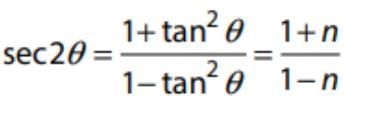
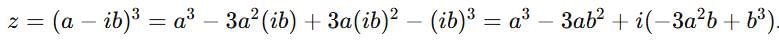
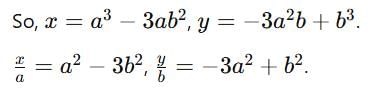
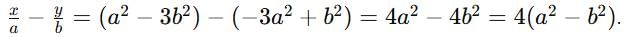
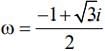 then (3 + ω + 3ω2)4 is
then (3 + ω + 3ω2)4 is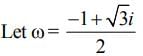
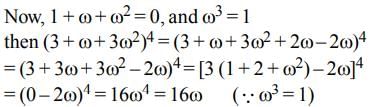
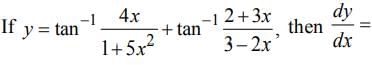
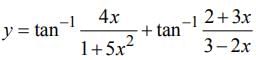
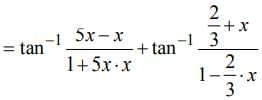
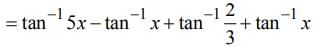
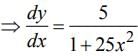
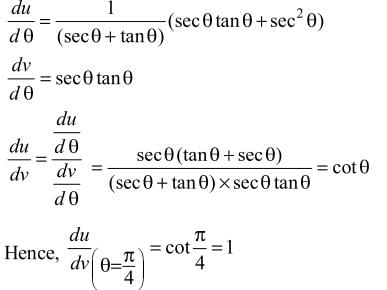
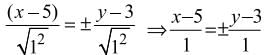
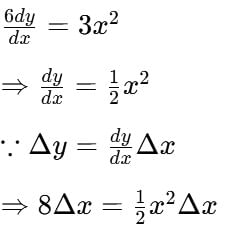
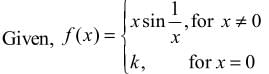
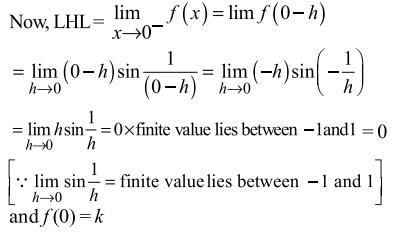
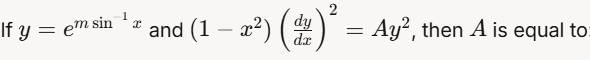
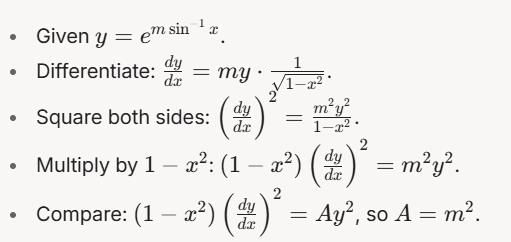
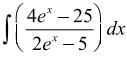 = Ax + Blog(2ex − 5) + C, then
= Ax + Blog(2ex − 5) + C, then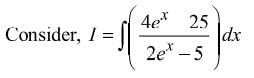
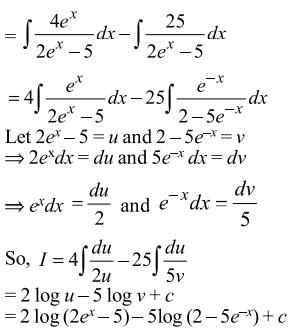
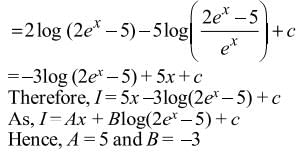
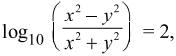 then dy/dx is equal to
then dy/dx is equal to