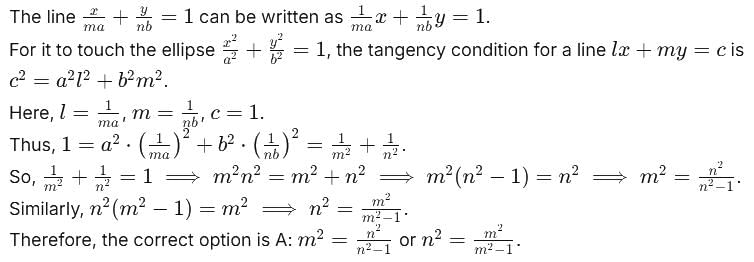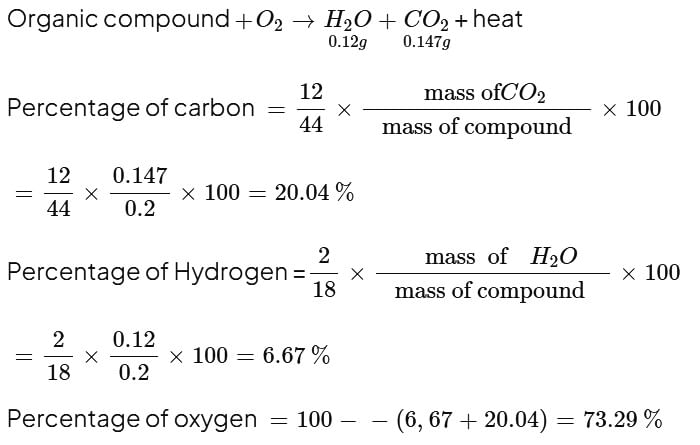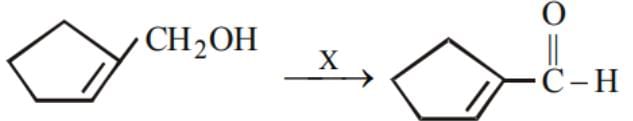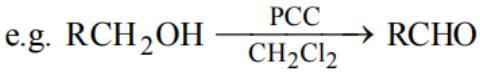UGEE SUPR Mock Test- 5 - JEE MCQ
30 Questions MCQ Test - UGEE SUPR Mock Test- 5
The electric potential V is given as a function of distance x (metre) by V = (5x2 + 10 x - 4) volt. Value of electric field at x = 1 m is
A proton moving with a velocity 3 x 105 m/senters a magnetic field of 0.3 tesla at an angle of 300 with the field. The radius of curvature of its path will be (e/m for proton = 108 C/kg)
If force (F), length (L) and time (T) are assumed to be fundamental units, then the dimensional formula of the mass will be
A metal disc of radius 100 cm is rotated at a constant angular speed of 60 rad/s in a plane at right angles to an external field of magnetic induction 0.05 Wb/m2. The emfinduced between the centre and a point on the rim will be
A balloon is rising vertically up with a velocityof 29 ms-1. A stone is dropped from it and it reaches the ground in 10 seconds. The height of the balloon when the stone dropped from it,was (g = 9.8 ms-2)
An alternating voltage V = V0 sin ωt is applied across a circuit. As a result, a current I = I0 sin(ωt π/2) flows in it. The power consumed percycle is
A gun fires two bullets at 600 and 300 with horizontal. The bullets strike at some horizontal distance. The ratio of maximum height for the two bullets is in the ratio of
In a simple pendulum of length I the bob is pulled aside from its equilibrium position through an angle θ and then released. The bob passes through the equilibrium position with speed
A moving coil galvanometer has resistance of 10Ω and full scale deflection of 0.01 A. It can be converted into voltmeter of 10 V full scale by connecting into resistance of
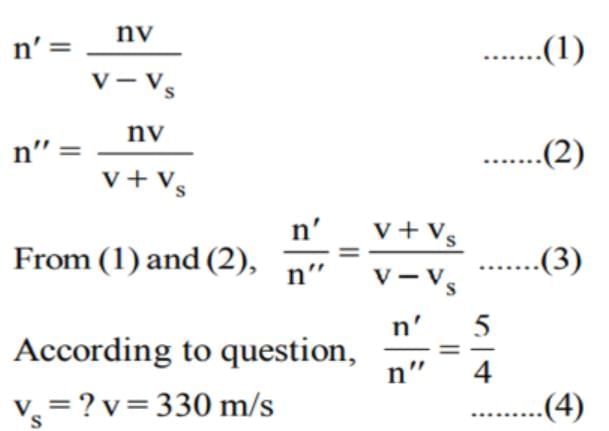
The frequency of whistle of an engine appears to be (4/5)th of initial frequency when it crosses a stationary observer. If the velocity of sound is 330 mis, then the speed of engine will be
Let [x] denote the greatest integer ≤ x. If f(x) = [x] and g (x) = lxl, then the value of
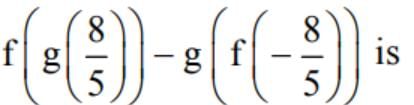
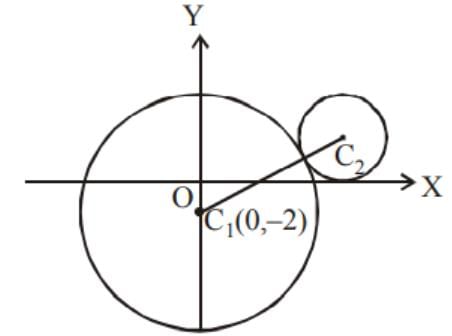
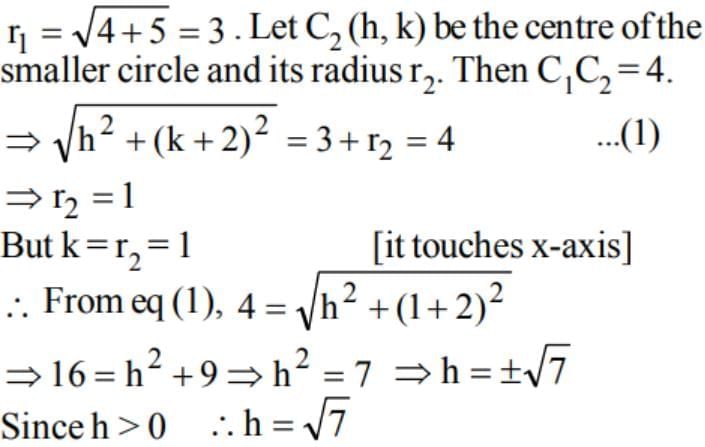
In the given figure, the equation of the larger circle is x2 + y2 + 4y - 5 = 0 and the distance between centres is 4. Then the equation of smaller circle is
The equation sin4 x - (k + 2) sin2 x - (k + 3) = 0 possesses a solution if
On reaction with sodium, 1 mol of a compound X gives 1 mol of H2. Which one of the following compounds might be X?
The number of real solutions of the equation
(x - 1)2 + (x - 2)2 + (x - 3)2 = 0 is
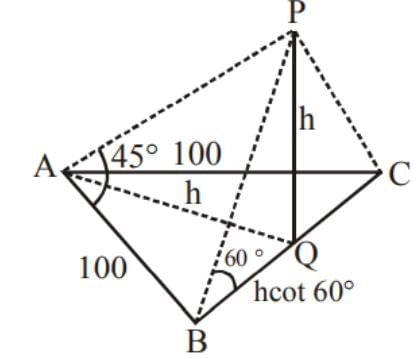
ABC is a triangular park with AB = AC = 100 m. A TV tower stands at the mid-point of BC. The angles of elevation of the top of the tower at A, B, C are 450, 600, 600 respectively. The height of the tower is
If a > 0, b > 0, c > 0 and a, b, c are distinct, then (a + b) (b + c) (c + a) is greater than
The equation ofthe tangent to the curve y = be-x/a at the point where it crosses they-axis is
If a, c, b are in GP., then the area of the triangle formed by the lines ax + by + c = 0 with the coordinates axes is equal to
Suppose that f(0) = -3 and f '(x) ≤ 5 for all values of x.
Then, the largest value which f(2) can attain is
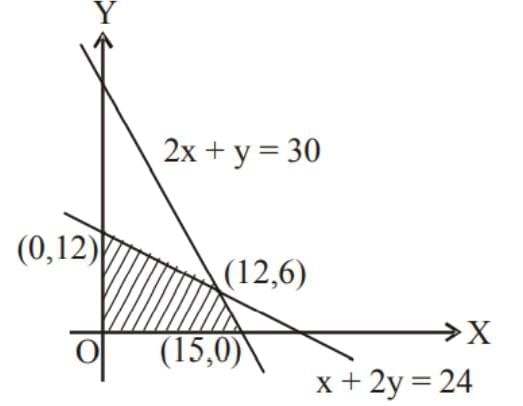
The maximum value of z = 6x + 8y subject to constraints 2x + y ≤ 30, x + 2y ≤ 24 and x ≥ 0, y ≥ 0 is
If 0.2 gram of an organic compound containing carbon, hydrogen and oxygen on combustion, yielded 0.147 gram carbon dioxide and 0.12 gram water, what will be the content of oxygen in substance?
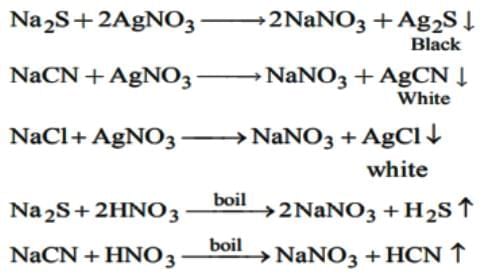
The Lassaigne's extract is boiled with dil. HNO3 before testing for halogens because


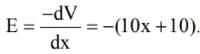
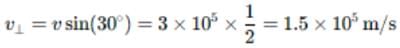
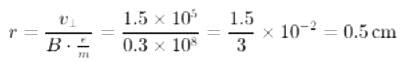

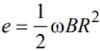
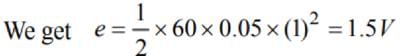
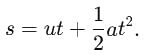
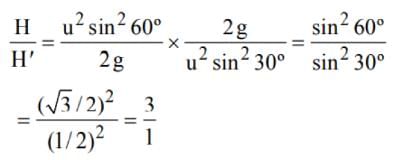
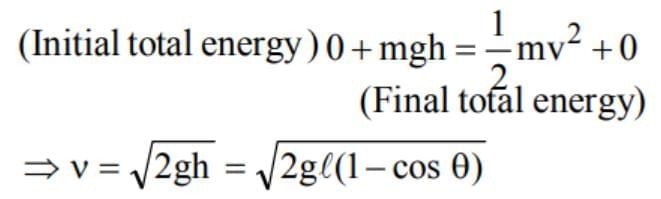
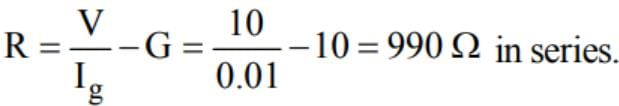

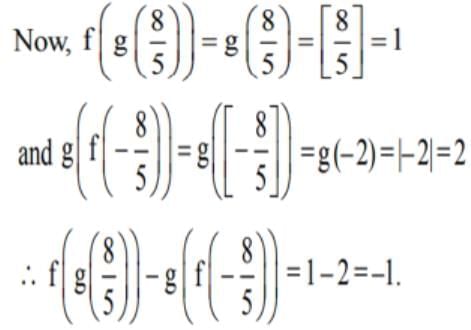
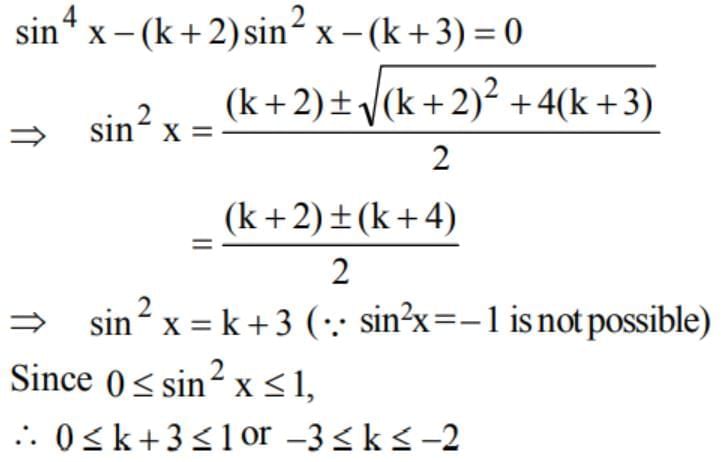
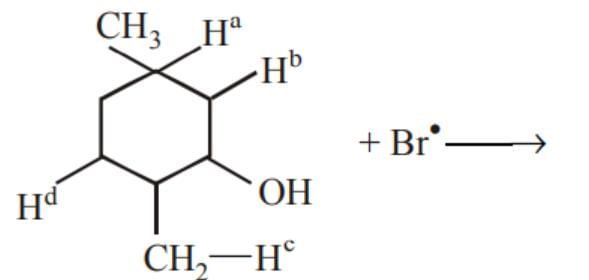
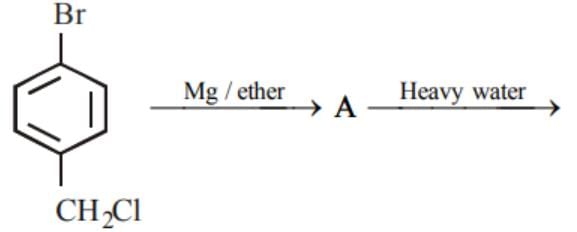
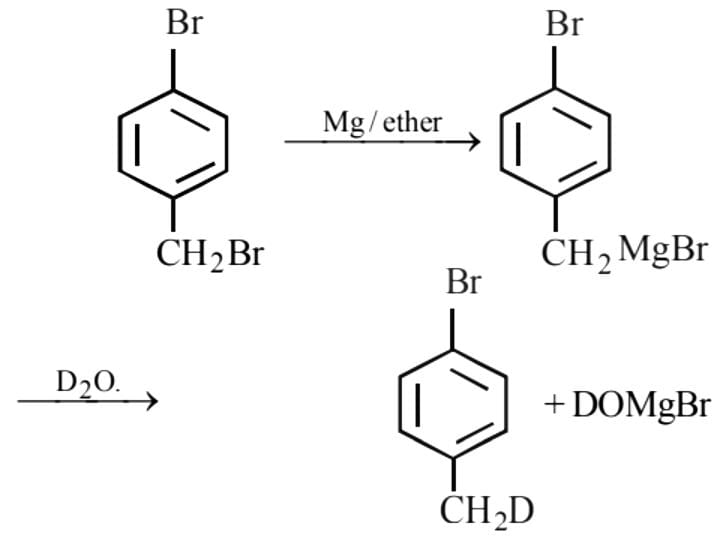
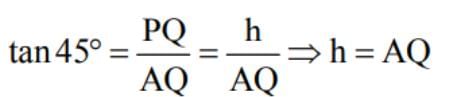
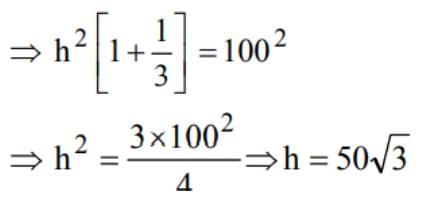
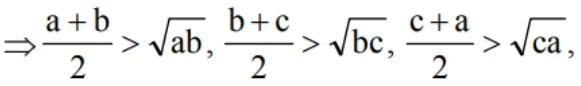
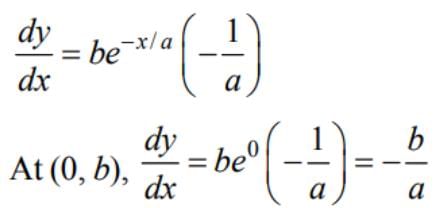
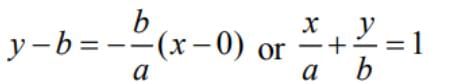
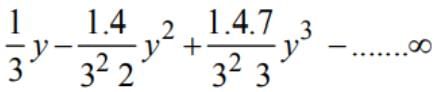 is equal to
is equal to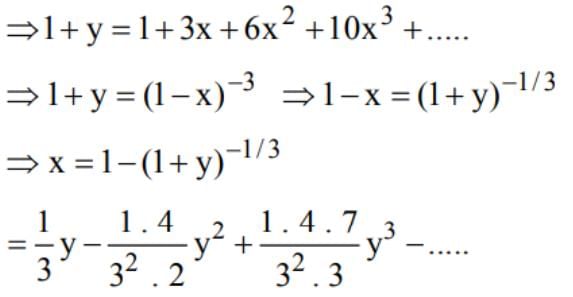
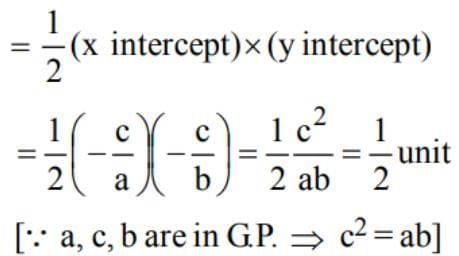
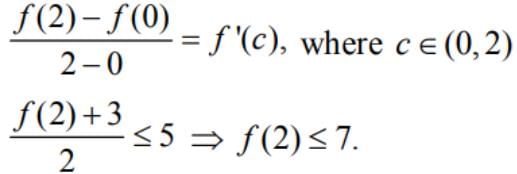
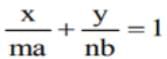 touches the ellipse
touches the ellipse 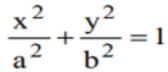 , then
, then