NEET Exam > NEET Tests > Chemistry Class 12 > Test: Ethers: Preparation & Properties - NEET MCQ
Test: Ethers: Preparation & Properties - NEET MCQ
Test Description
10 Questions MCQ Test Chemistry Class 12 - Test: Ethers: Preparation & Properties
Test: Ethers: Preparation & Properties for NEET 2025 is part of Chemistry Class 12 preparation. The Test: Ethers: Preparation & Properties questions and answers have been
prepared according to the NEET exam syllabus.The Test: Ethers: Preparation & Properties MCQs are made for NEET 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Ethers: Preparation & Properties below.
Solutions of Test: Ethers: Preparation & Properties questions in English are available as part of our Chemistry Class 12 for NEET & Test: Ethers: Preparation & Properties solutions in
Hindi for Chemistry Class 12 course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Ethers: Preparation & Properties | 10 questions in 15 minutes | Mock test for NEET preparation | Free important questions MCQ to study Chemistry Class 12 for NEET Exam | Download free PDF with solutions
Detailed Solution for Test: Ethers: Preparation & Properties - Question 1
Test: Ethers: Preparation & Properties - Question 2
Ethers may be used as solvents because they react only with which of the following reactants?
Detailed Solution for Test: Ethers: Preparation & Properties - Question 2
Detailed Solution for Test: Ethers: Preparation & Properties - Question 3
Test: Ethers: Preparation & Properties - Question 4
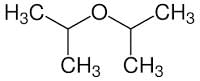
What is the IUPAC name of di-isopropyl ether
Detailed Solution for Test: Ethers: Preparation & Properties - Question 4
Detailed Solution for Test: Ethers: Preparation & Properties - Question 5
Test: Ethers: Preparation & Properties - Question 6
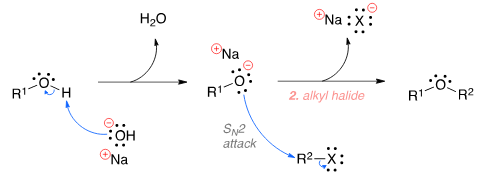
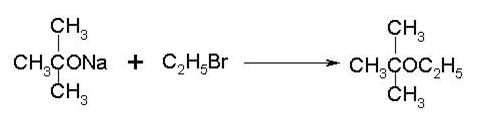
To prepare tert-butyl ethyl ether, the reagents required are:
Detailed Solution for Test: Ethers: Preparation & Properties - Question 6
Detailed Solution for Test: Ethers: Preparation & Properties - Question 7
Test: Ethers: Preparation & Properties - Question 8
Which of the following is an example of a symmetrical ether?
Detailed Solution for Test: Ethers: Preparation & Properties - Question 8
Test: Ethers: Preparation & Properties - Question 9
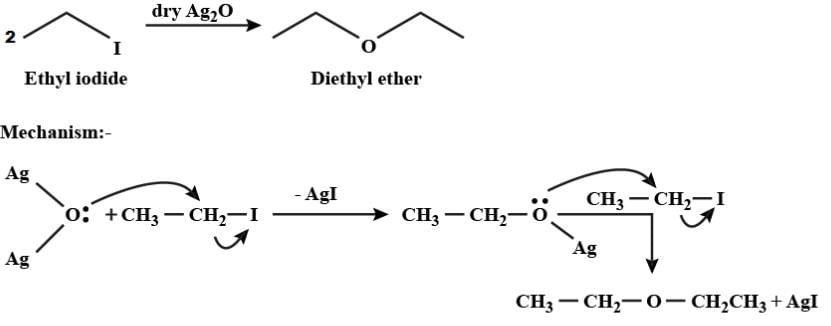
When ethyl iodide reacts with dry silver oxide, the product formed is:
Detailed Solution for Test: Ethers: Preparation & Properties - Question 9
Test: Ethers: Preparation & Properties - Question 10
What type of hybridization is present in the oxygen atom of ethers?
Detailed Solution for Test: Ethers: Preparation & Properties - Question 10
|
108 videos|286 docs|123 tests
|
Information about Test: Ethers: Preparation & Properties Page
In this test you can find the Exam questions for Test: Ethers: Preparation & Properties solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Ethers: Preparation & Properties, EduRev gives you an ample number of Online tests for practice