Test: Coordination Compounds - 1 - NEET MCQ
25 Questions MCQ Test Chemistry Class 12 - Test: Coordination Compounds - 1
Which among the following is an ambidentate ligand?
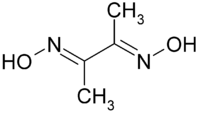
Which of the following represents chelating ligand?
The coordination number of Cr in [Cr (NH3)3 (H2O)3]
Which of the following complex will give white precipitate with barium chloride solution?
In which of the following complexes, the nickel metal is in the highest oxidation state?
Which of the following can be termed as mixed complex?
Which of the following ligand gives chelate complexes?
In the formation of complex entity, the central atom/ion acts as
Sodium pentacyanonitrosylferrate(II) is also called?
Which among the following has square pyramidal geometry?
The hybridization of nickel in tetracarbonyl nickel is
According to Werner’s theory , the primary valences of the central atom
In the complex PtCl4.3NH3 the number of ionisable chlorines is
|
75 videos|338 docs|78 tests
|