NEET Exam > NEET Tests > NCERT Based Tests for NEET > Test: Group-16 Elements - NEET MCQ
Test: Group-16 Elements - NEET MCQ
Test Description
5 Questions MCQ Test NCERT Based Tests for NEET - Test: Group-16 Elements
Test: Group-16 Elements for NEET 2025 is part of NCERT Based Tests for NEET preparation. The Test: Group-16 Elements questions and answers have been
prepared according to the NEET exam syllabus.The Test: Group-16 Elements MCQs are made for NEET 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Group-16 Elements below.
Solutions of Test: Group-16 Elements questions in English are available as part of our NCERT Based Tests for NEET for NEET & Test: Group-16 Elements solutions in
Hindi for NCERT Based Tests for NEET course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Group-16 Elements | 5 questions in 5 minutes | Mock test for NEET preparation | Free important questions MCQ to study NCERT Based Tests for NEET for NEET Exam | Download free PDF with solutions
Test: Group-16 Elements - Question 1
Group 16 elements have a lower value of first ionization enthalpy as compared to group 15 elements because:
Detailed Solution for Test: Group-16 Elements - Question 1
Test: Group-16 Elements - Question 2
Covalency of oxygen cannot exceed 2 unlike sulphur which can show +4 or +6 because
Detailed Solution for Test: Group-16 Elements - Question 2
Test: Group-16 Elements - Question 3
Bond angle in H2O(104.5∘) is higher than the bond angle of H2S (92.1∘). The difference is due to:
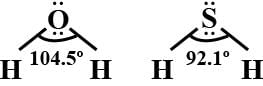
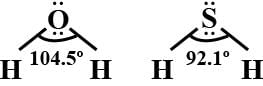
Detailed Solution for Test: Group-16 Elements - Question 3
Test: Group-16 Elements - Question 4
Arrange the following hydrides of group 16 elements in order of increasing stability:
Detailed Solution for Test: Group-16 Elements - Question 4
|
684 tests
|
Information about Test: Group-16 Elements Page
In this test you can find the Exam questions for Test: Group-16 Elements solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Group-16 Elements, EduRev gives you an ample number of Online tests for practice