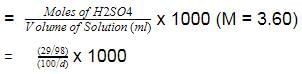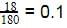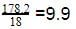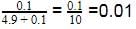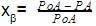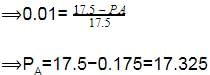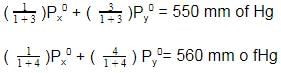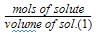Test: JEE Previous Year Questions- Solutions - JEE MCQ
26 Questions MCQ Test Chapter-wise Tests for JEE Main & Advanced - Test: JEE Previous Year Questions- Solutions
Which of the following concentration factor is affected by change in temperature ?
[AIEEE-2002]
For an aqueous solution, freezing point is _0.186ºC . Boiling point of the same solution is
(Kƒ = 1.86º K mol-1 kg) and Kb = 0.512º K mol-1 kg)
[AIEEE-2002]
In a mixture of A and B, components show negative deviation when [AIEEE-2002]
A pressure cooker reduces cooking time for food because _
[AIEEE-2003]
If liquids A and B form an ideal solution _
[AIEEE-2003]
In a 0.2 molal aqueous solution of a weak acid HX the degree of ionization is 0.3 . Taking kƒ for water as 1.85, the freezing point of the solution will be nearest to _
[AIEEE-2003]
If liquid A and B form ideal solution, than
[AIEEE-2003]
Which one of the following aqueous solutions will exhibit highest boiling point ?
[AIEEE-2004]
Osmotic pressure of 40% (wt./vol.) urea solution is 1.64 atm and that of 3.42% (wt./vol.) cane sugar is 2.46 atm. When equal volumes of the above two solutions are mixed, the osmotic pressure of the resulting solution is -
Which of the following liquid pairs shows a positive deviation from Raoult's law ?
[AIEEE-2004]
Which one of the following statement is false?
[AIEEE-2004]
If a is the degree of dissociation of Na2SO4, the vant Hoff's factor (i) used for calculating the molecular mass is–
[AIEEE-2005]
Benzene and toluene form nearly ideal solutions. At 20ºC, the vapour pressure of benzene is 75 torr and that of toluene is 22 torr. The partial pressure of benzene at 20ºC for a solution containing 78 g of benzene and 46 g of toluene in torr is
[AIEEE-2005]
Two solutions of a substance (non electrolyte) are mixed in the following manner. 480 ml of 1.5 M first solution + 520 mL of 1.2 M second solution. What is the molarity of the final mixture ?
[AIEEE-2005]
Which one of the following aqueous solutions will exhibit highest boiling point?
18 g of glucose (C6H12O6) is added to 178.2 g of water. The vapour pressure of water for this aqueous solution at 100º C is -
[AIEEE 2006]
Density of a 2.05 M solution of acetic acid in water is 1.02 g/mL. The molality of the solution is -
[AIEEE 2006]
A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be -
[AIEEE 2007]
A 5.25% solution of a substance is isotonic with a 1.5% solution of urea (molar mass = 60 g mol-1) in the same solvent. If the densities of both the solutions are assumed to be equal to 1.0 gcm-3, molar mass of the substance will be-
[AIEEE 2007]
The density (in g mL-1) of a 3.60 M sulphuric acid solution that is 29% H2SO4(Molar mass = 98 g mol-1) by mass will be -
[AIEEE 2007]
The vapour pressure of water at 20º C is 17.5 mm Hg. If 18g of glucose (C6H12O6) is added to 178.2 g of water at 20° C, the vapour pressure of the resulting solution will be –
[AIEEE 2008]
At 80º C , the vapour pressure of pure liquid `A' is 520 mm Hg and that of pure liquid `B' is 1000 mm Hg. If a mixture solution of `A' and `B' boils at 80º C and 1 atm pressure, the amount of `A' in the mixture is (1 atm = 760 mm Hg)
[AIEEE 2008]
A binary liquid solution is prepared by mixing n-heptane and ethanol. Which one of the following statements is correct regarding the behaviour of the solution ?
[AIEEE 2009]
Two liquids X and Y form an ideal solution At 300 K, vapour pressure of the solution containing 1 mol of X and 3 mol of Y is 550 mmHg. At the same temperature, if 1 mol of Y is further added to this solution, vapour pressure of the solution increases by 10 mmHg. Vapour pressure (in mmHg) of X and Y in their pure states will be, respectively -
[AIEEE 2009]
Kf for water is 1.86 K kg mol-1. If your automobile radiator holds 1.0 kg of water, how many grams of ethylene glycol (C2H6O2) must you add to get the freezing point of the solution lowered to – 2.8°C ?
[AIEEE-2012]
The density of a solution prepared by dissolving 120 g of urea (mol. mass = 60 u) in 1000g of water is 1.15 g/mL. The molarity of this solution is :
[AIEEE-2012]
|
446 docs|930 tests
|




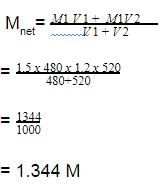
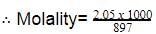
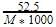 The molar concentration of urea solution is
The molar concentration of urea solution is 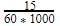
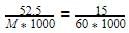
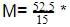 60 = 210.0 g/mol.
60 = 210.0 g/mol.