Test: Atoms- Case Based Type Questions - Grade 12 MCQ
10 Questions MCQ Test - Test: Atoms- Case Based Type Questions
Read the following text and answer the following questions on the basis of the same:
Atomic Absorption Spectrometer: The atomic absorption (AA) spectrometer is used to analyze metals at very low concentrations, typically in the parts per million (ppm) or parts per billion (ppb) ranges. A liquid sample containing dissolved material whose concentration is to be measured is aspirated into a thin, wide AA flame, or is introduced into a small carbon furnace which is heated to a high temperature.
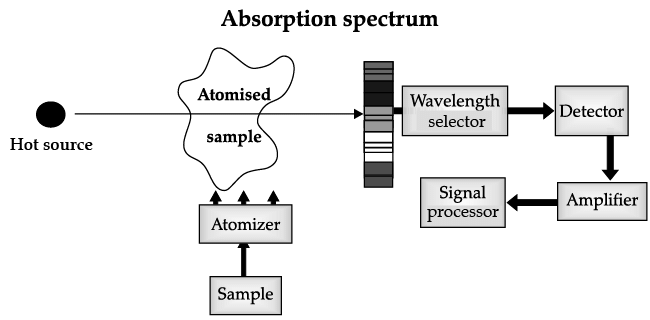
Basic Principle of AAS is the measurement of absorption of radiation by free atoms. The total amount of absorption depends on the number of free atoms present and the degree to which the free atoms absorb the radiation. At the high temperature of the AA flame, the sample is broken down into atoms using an atomizer and it is the concentration of these atoms that is measured.
Sample in the form of solution is used. It is broken up into a fine mist with the help of an atomizer.
When the mist reaches the flame, the intense heat breaks up the sample into its individual atoms.
When a photon coming out from the hot source hits an atom and the energy of the photon is equal to the gap between two electron energy levels of the atom, then the electron in the lower energy level absorb the photon and jumps up to the higher energy level. If the photon energy does not correspond to the difference between two energy levels, then the photon will not be absorbed (it may be scattered away).
Hence in the spectrum, the wavelength corresponding to the absorbed photons is observed as black lines as shown in the following spectrum of Hydrogen. The dark lines correspond to the frequencies of light those have been absorbed by the sample element.

Using this process, a source of photons (generally a white light) of various energies is used to obtain the absorption spectra of different materials and to identify them.
Q. What happens when a photon hits an atom and the energy of the photon is not equal to the gap between two electron energy levels of the atom?

Read the following text and answer the following questions on the basis of the same:
Atomic Absorption Spectrometer: The atomic absorption (AA) spectrometer is used to analyze metals at very low concentrations, typically in the parts per million (ppm) or parts per billion (ppb) ranges. A liquid sample containing dissolved material whose concentration is to be measured is aspirated into a thin, wide AA flame, or is introduced into a small carbon furnace which is heated to a high temperature.
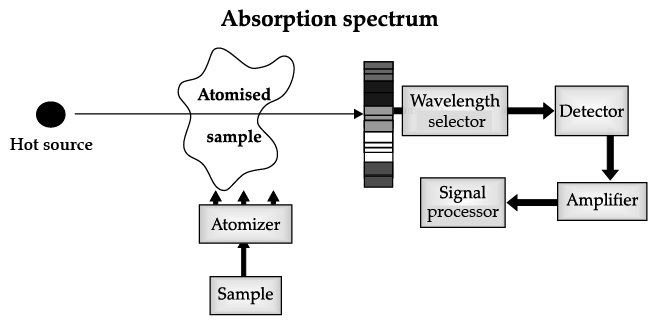
Basic Principle of AAS is the measurement of absorption of radiation by free atoms. The total amount of absorption depends on the number of free atoms present and the degree to which the free atoms absorb the radiation. At the high temperature of the AA flame, the sample is broken down into atoms using an atomizer and it is the concentration of these atoms that is measured.
Sample in the form of solution is used. It is broken up into a fine mist with the help of an atomizer.
When the mist reaches the flame, the intense heat breaks up the sample into its individual atoms.
When a photon coming out from the hot source hits an atom and the energy of the photon is equal to the gap between two electron energy levels of the atom, then the electron in the lower energy level absorb the photon and jumps up to the higher energy level. If the photon energy does not correspond to the difference between two energy levels, then the photon will not be absorbed (it may be scattered away).
Hence in the spectrum, the wavelength corresponding to the absorbed photons is observed as black lines as shown in the following spectrum of Hydrogen. The dark lines correspond to the frequencies of light those have been absorbed by the sample element.

Using this process, a source of photons (generally a white light) of various energies is used to obtain the absorption spectra of different materials and to identify them.
Q. What should be the concentration of metal for analysis using Atomic Absorption Spectrometer?

Read the following text and answer the following questions on the basis of the same:
Atomic Absorption Spectrometer: The atomic absorption (AA) spectrometer is used to analyze metals at very low concentrations, typically in the parts per million (ppm) or parts per billion (ppb) ranges. A liquid sample containing dissolved material whose concentration is to be measured is aspirated into a thin, wide AA flame, or is introduced into a small carbon furnace which is heated to a high temperature.
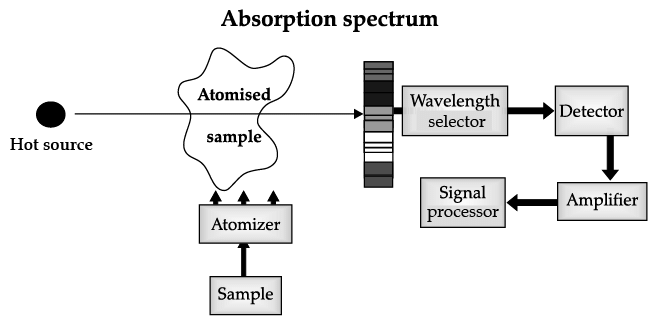
Basic Principle of AAS is the measurement of absorption of radiation by free atoms. The total amount of absorption depends on the number of free atoms present and the degree to which the free atoms absorb the radiation. At the high temperature of the AA flame, the sample is broken down into atoms using an atomizer and it is the concentration of these atoms that is measured.
Sample in the form of solution is used. It is broken up into a fine mist with the help of an atomizer.
When the mist reaches the flame, the intense heat breaks up the sample into its individual atoms.
When a photon coming out from the hot source hits an atom and the energy of the photon is equal to the gap between two electron energy levels of the atom, then the electron in the lower energy level absorb the photon and jumps up to the higher energy level. If the photon energy does not correspond to the difference between two energy levels, then the photon will not be absorbed (it may be scattered away).
Hence in the spectrum, the wavelength corresponding to the absorbed photons is observed as black lines as shown in the following spectrum of Hydrogen. The dark lines correspond to the frequencies of light those have been absorbed by the sample element.

Using this process, a source of photons (generally a white light) of various energies is used to obtain the absorption spectra of different materials and to identify them.
Q. What is the basic principle of Atomic Absorption Spectrophotometer?

Read the following text and answer the following questions on the basis of the same:
Atomic Absorption Spectrometer: The atomic absorption (AA) spectrometer is used to analyze metals at very low concentrations, typically in the parts per million (ppm) or parts per billion (ppb) ranges. A liquid sample containing dissolved material whose concentration is to be measured is aspirated into a thin, wide AA flame, or is introduced into a small carbon furnace which is heated to a high temperature.
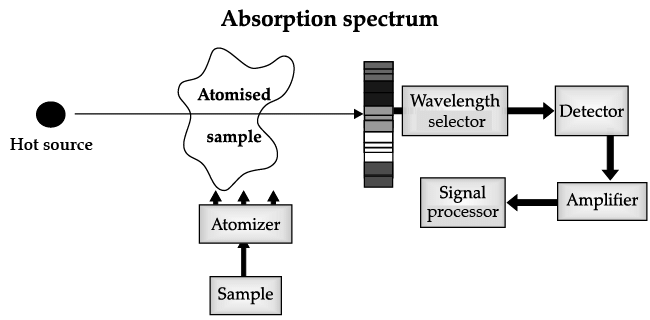
Basic Principle of AAS is the measurement of absorption of radiation by free atoms. The total amount of absorption depends on the number of free atoms present and the degree to which the free atoms absorb the radiation. At the high temperature of the AA flame, the sample is broken down into atoms using an atomizer and it is the concentration of these atoms that is measured.
Sample in the form of solution is used. It is broken up into a fine mist with the help of an atomizer.
When the mist reaches the flame, the intense heat breaks up the sample into its individual atoms.
When a photon coming out from the hot source hits an atom and the energy of the photon is equal to the gap between two electron energy levels of the atom, then the electron in the lower energy level absorb the photon and jumps up to the higher energy level. If the photon energy does not correspond to the difference between two energy levels, then the photon will not be absorbed (it may be scattered away).
Hence in the spectrum, the wavelength corresponding to the absorbed photons is observed as black lines as shown in the following spectrum of Hydrogen. The dark lines correspond to the frequencies of light those have been absorbed by the sample element.
![]()
Using this process, a source of photons (generally a white light) of various energies is used to obtain the absorption spectra of different materials and to identify them.
Q. How the corresponding wavelength of the absorbed photon is represented in the absorption spectrum?
Read the following text and answer the following questions on the basis of the same:
Atomic Absorption Spectrometer: The atomic absorption (AA) spectrometer is used to analyze metals at very low concentrations, typically in the parts per million (ppm) or parts per billion (ppb) ranges. A liquid sample containing dissolved material whose concentration is to be measured is aspirated into a thin, wide AA flame, or is introduced into a small carbon furnace which is heated to a high temperature.
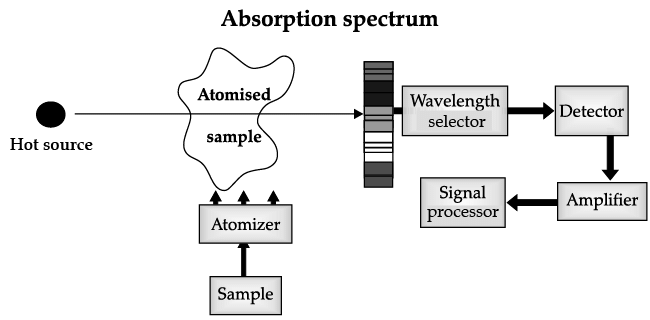
Basic Principle of AAS is the measurement of absorption of radiation by free atoms. The total amount of absorption depends on the number of free atoms present and the degree to which the free atoms absorb the radiation. At the high temperature of the AA flame, the sample is broken down into atoms using an atomizer and it is the concentration of these atoms that is measured.
Sample in the form of solution is used. It is broken up into a fine mist with the help of an atomizer.
When the mist reaches the flame, the intense heat breaks up the sample into its individual atoms.
When a photon coming out from the hot source hits an atom and the energy of the photon is equal to the gap between two electron energy levels of the atom, then the electron in the lower energy level absorb the photon and jumps up to the higher energy level. If the photon energy does not correspond to the difference between two energy levels, then the photon will not be absorbed (it may be scattered away).
Hence in the spectrum, the wavelength corresponding to the absorbed photons is observed as black lines as shown in the following spectrum of Hydrogen. The dark lines correspond to the frequencies of light those have been absorbed by the sample element.
![]()
Using this process, a source of photons (generally a white light) of various energies is used to obtain the absorption spectra of different materials and to identify them.
Q. How the sample for analysis is driven to atomic state in AAS?
Read the following text and answer the following questions on the basis of the same:
Spectrum Analysis and Astronomy
Each element in the periodic table can appear in gaseous form and produce its own spectrum unique to that element. Hydrogen will not look like Helium, which will not look like carbon which will not look like iron... and soon.
Astrophysicists can identify what kinds of materials are present in stars from the analysis of star’s spectra. This type of study is called astronomical spectroscopy.
The science of spectroscopy is quite sophisticated. From spectrum lines analysis astrophysicists can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star.
The width of the line can tell us how fast the material is moving. We can learn about winds in stars from this. The shifting of spectral lines shift back and forth indicates that the star may be orbiting another star.
The following table shows a rough guide for the relationship between the temperature of a star and the electromagnetic spectrum.
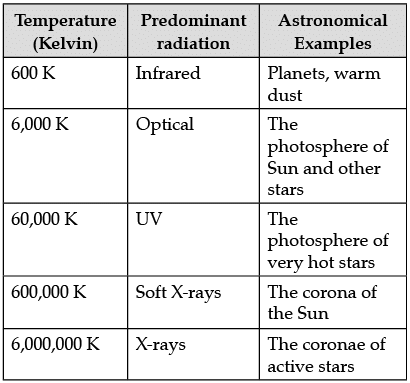
If the spectrum of a star is red or blue shifted, then it can be used to infer its velocity along the line of sight. Edwin Hubble observed that more distant galaxies tended to have more red shifted spectra. This establishes the theory of expansion of the universe.
Q. From the spectrum analysis the following information of a star can be obtained.
Read the following text and answer the following questions on the basis of the same:
Spectrum Analysis and Astronomy
Each element in the periodic table can appear in gaseous form and produce its own spectrum unique to that element. Hydrogen will not look like Helium, which will not look like carbon which will not look like iron... and soon.
Astrophysicists can identify what kinds of materials are present in stars from the analysis of star’s spectra. This type of study is called astronomical spectroscopy.
The science of spectroscopy is quite sophisticated. From spectrum lines analysis astrophysicists can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star.
The width of the line can tell us how fast the material is moving. We can learn about winds in stars from this. The shifting of spectral lines shift back and forth indicates that the star may be orbiting another star.
The following table shows a rough guide for the relationship between the temperature of a star and the electromagnetic spectrum.
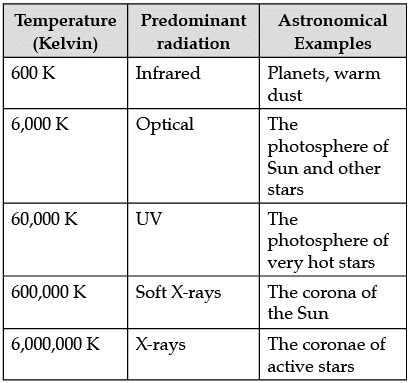
If the spectrum of a star is red or blue shifted, then it can be used to infer its velocity along the line of sight. Edwin Hubble observed that more distant galaxies tended to have more red shifted spectra. This establishes the theory of expansion of the universe.
Q. What may be the approximate temperature if soft X-rays are found predominantly in the spectrum?
Read the following text and answer the following questions on the basis of the same:
Spectrum Analysis and Astronomy
Each element in the periodic table can appear in gaseous form and produce its own spectrum unique to that element. Hydrogen will not look like Helium, which will not look like carbon which will not look like iron... and soon.
Astrophysicists can identify what kinds of materials are present in stars from the analysis of star’s spectra. This type of study is called astronomical spectroscopy.
The science of spectroscopy is quite sophisticated. From spectrum lines analysis astrophysicists can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star.
The width of the line can tell us how fast the material is moving. We can learn about winds in stars from this. The shifting of spectral lines shift back and forth indicates that the star may be orbiting another star.
The following table shows a rough guide for the relationship between the temperature of a star and the electromagnetic spectrum.
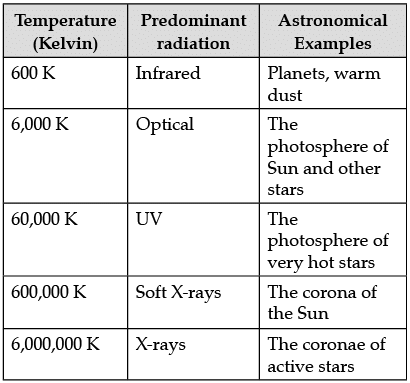
If the spectrum of a star is red or blue shifted, then it can be used to infer its velocity along the line of sight. Edwin Hubble observed that more distant galaxies tended to have more red shifted spectra. This establishes the theory of expansion of the universe.
Q. What is astronomical spectroscopy?
Read the following text and answer the following questions on the basis of the same:
Spectrum Analysis and Astronomy
Each element in the periodic table can appear in gaseous form and produce its own spectrum unique to that element. Hydrogen will not look like Helium, which will not look like carbon which will not look like iron... and soon.
Astrophysicists can identify what kinds of materials are present in stars from the analysis of star’s spectra. This type of study is called astronomical spectroscopy.
The science of spectroscopy is quite sophisticated. From spectrum lines analysis astrophysicists can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star.
The width of the line can tell us how fast the material is moving. We can learn about winds in stars from this. The shifting of spectral lines shift back and forth indicates that the star may be orbiting another star.
The following table shows a rough guide for the relationship between the temperature of a star and the electromagnetic spectrum.

If the spectrum of a star is red or blue shifted, then it can be used to infer its velocity along the line of sight. Edwin Hubble observed that more distant galaxies tended to have more red shifted spectra. This establishes the theory of expansion of the universe.
Q. The lines in a star ’s spectrum is found to shift back and forth. What conclusion may be drawn from this observation?
Read the following text and answer the following questions on the basis of the same:
Spectrum Analysis and Astronomy
Each element in the periodic table can appear in gaseous form and produce its own spectrum unique to that element. Hydrogen will not look like Helium, which will not look like carbon which will not look like iron... and soon.
Astrophysicists can identify what kinds of materials are present in stars from the analysis of star’s spectra. This type of study is called astronomical spectroscopy.
The science of spectroscopy is quite sophisticated. From spectrum lines analysis astrophysicists can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star.
The width of the line can tell us how fast the material is moving. We can learn about winds in stars from this. The shifting of spectral lines shift back and forth indicates that the star may be orbiting another star.
The following table shows a rough guide for the relationship between the temperature of a star and the electromagnetic spectrum.
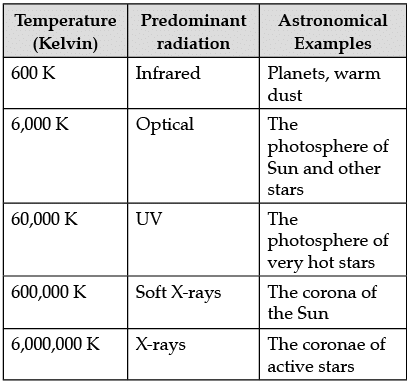
If the spectrum of a star is red or blue shifted, then it can be used to infer its velocity along the line of sight. Edwin Hubble observed that more distant galaxies tended to have more red shifted spectra. This establishes the theory of expansion of the universe.
Q. Which nature of spectrum establishes the theory of the expanding universe?



















