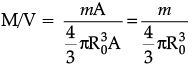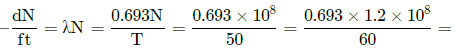Assertion & Reason Test: Nuclei - Grade 12 MCQ
10 Questions MCQ Test - Assertion & Reason Test: Nuclei
Directions : In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Nuclear force is same between neutron-neutron, proton-proton and neutron proton.
Reason (R): Nuclear force is charge independent.
Directions : In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): The binding energy per nucleon, for nuclei with mass number (A) > 56 decreases with A.
Reason (R): Nuclear force is weak in heavier nuclei.
Assertion (A) : The isotopes are the atoms of the same element with different atomic masses. They require different positions in the periodic table.
Reason (R) : Variable number of neutrons in the nuclei of the atoms of same elements leads to the different atomic masses.
Reason (R) : Variable number of neutrons in the nuclei of the atoms of same elements leads to the different atomic masses.
Directions : In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Electrons do not experience strong nuclear force.
Reason (R): Strong nuclear force is charge independent.
Directions : In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Density of all the nuclei is same.
Reason (R): Radius of nucleus is directly proportional to the cube root of mass number.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : Density of all the nuclei is the same.
Reason : Radius of nucleus is directly proportional to the cube root of mass number.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : The mass number of a nucleus is always less than its atomic number.
Reason : Mass number of a nucleus may be equal to its atomic number.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : Radioactivity of 108 undecayed radioactive nuclei of half life of 50 days is equal to that of 1.2 × 108 number of undecayed nuclei of some other material with half life of 60 days.
Reason : Radioactivity is proportional to half-life.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : Radioactive nuclei emit β–1 particles.
Reason : Electrons exist inside the nucleus.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : The heavier nuclei tend to have larger N/Z ratio because neutrons do not exert electric force.
Reason : Coulomb forces have longer range compared to the nuclear force.