31 Year NEET Previous Year Questions: Kinetic Theory - NEET MCQ
19 Questions MCQ Test - 31 Year NEET Previous Year Questions: Kinetic Theory
Two containers A and B are partly filled with water and closed. The volume of A is twice that of B and it contains half the amount of water in B. If both are at the same temperature, the water vapour in the containers will have pressure in the ratio of [1988]
At constant volume, temperature is increased then
A polyatomic gas with n degrees of freedom has a mean energy per molecule given by [1989]
For a certain gas the ratio of specific heats is given to be γ = 1.5. For this gas [1990]
According to kinetic theory of gases, at absolute zero temperature [1990]
One mole of an ideal monoatomic gas requires 207 J heat to raise the temperature by 10 K when heated at constant pressure. If the same gas is heated at constant volume to raise the temperature by the same 10 K, the heat required is [Given the gas constant R = 8.3 J/ mol. K] [1990]
Relation between pressure (P) and energy (E) of a gas is [1991]
Three containers of the same volume contain three different gases. The masses of the molecules are m1, m2 and m3 and the number of molecules in their respective containers are N1, N2 and N3. The gas pressure in the containers are P1, P2 and P3 respectively. All the gases are now mixed and put in one of these containers.The pressure P of the mixture will be [1991]
The number of translational degrees of freedom for a diatomic gas is [1993]
The temperature of a gas is raised from 27°C to 927°C.The root mean square speed is [1994]
The equation of state, corresponding to 8g of O2 is[1994]
If Cs be the velocity of sound in air and C be the r.m.s velocity, then [1994]
At 0 K, which of the following properties of a gas will be zero? [1996]
The degree of freedom of a molecule of a triatomic ga s is[1999]
The equation of state for 5 g of oxygen at a pressure P and temperature T, when occupying a volume V, will be [2004]
At 10° C the value of the density of a fixed mass of an ideal gas divided by its pressure is x. At 110°C this ratio is: [2008]
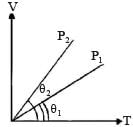
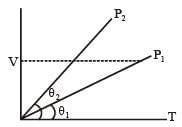
In the given (V – T) diagram, what is the relation between pressure P1 and P2 ? [NEET 2013]
The amount of heat energy required to raise the temperature of 1g of Helium at NTP, from T1K to T2K is [NEET 2013]
In a vessel, the gas is at a pressure P. If the mass of all the molecules is halved and their speed is doubled, then the resultant pressure will be [NEET Kar. 2013]



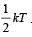 For a polyatomic gas with n degrees of freedom, the mean energy per molecule =
For a polyatomic gas with n degrees of freedom, the mean energy per molecule =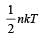
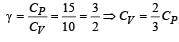
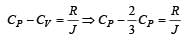
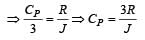
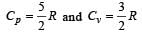
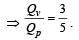
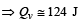
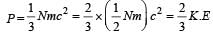
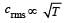
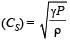
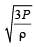
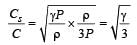
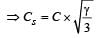
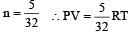 [∵ PV = nRT]
[∵ PV = nRT]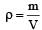
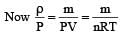
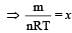 (By question)
(By question)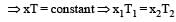
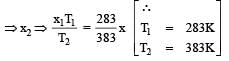
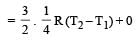
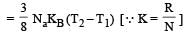
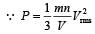
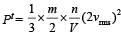 = 2 P.
= 2 P.















