Test: Electronic Configuration of Elements (May 9) - NEET MCQ
10 Questions MCQ Test - Test: Electronic Configuration of Elements (May 9)
Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?
Which of the following elements shown as pairs with their atomic numbers belong to the same period?
To which group,an element with atomic number 88 will belong?
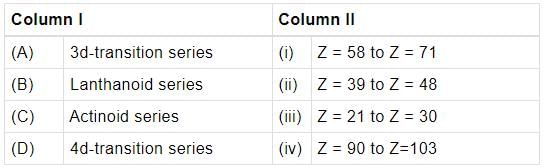
Match the column I with column II and mark the appropriate choice.
An element has atomic number 79. Predict the group and period in which the element is placed.
Atomic numbers of few elements are given below. Which of the pairs belongs to s-block?
An element has the electronic configuration
1s2 2s2 2p6 3s2 3p6 3d8 4s2.
What will be its position in.the periodic table?
Examples of elements belonging to s, p, d or f-block are given below. Identify the wrong example,
Few general names are given along with their valence shell configurations. Mark the incorrect name.
Electronic configuration of four elements is given below. Which of the following does not belong to the same group?














