Important Questions (1 mark): Matter in our Surroundings - UPSC MCQ
25 Questions MCQ Test General Science(Prelims) by IRS Divey Sethi - Important Questions (1 mark): Matter in our Surroundings
The room temperature on Celsius scale is 25°C. What is the temperature on the Kelvin scale?
The melting points of two solids [A] and [B] are 300K and 350K respectively. Which has more inter-particles forces?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following is not endothermic process?
The two major gases present in air are
Statement A: In Kelvin temperature, the symbol (∘) is not used.
Statement B: Mercury is used in glass thermometers because it does not stick with glass.
Which of the two statements is true?
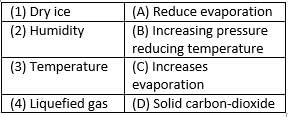
Match the following with correct response.
Which of the following solids undergo sublimation upon heating?
Which of the following is not a compound?
Name one common substance which can undergo a change in state upon heating or cooling.
Rate of diffusion is the fastest in
When the liquid starts boiling, the further heat energy which is supplied
Sulphur dioxide is collected in the lab in a gas jar by upward displacement of air. Which of the following statement is correct regarding the density of the gas?
Pressure on the surface of a gas is increased. What will happen to the inter-particle forces?
What are the characteristics of the particles of matter?
A. Particles of matter are in continuous motion
B. Particles of matters do not have spaces between them
C. Particles of matter attract each other
D. Particles of matter are very large in size
Statement A: Evaporation is a surface phenomenon but boiling is not.
Statement B: The diffusion of the gases varies inversely as the square root of their densities.
Which of the two statements is true?
Statement A: The temperature of the liquid becomes constant once it starts boiling
Statement B: Pressure of air at sea level is 70cm
Which of the two statements is true?
In which one of the following sets is each one a solid under ordinary conditions-
A substance upon heating directly changes into gaseous state. What is this change called?
Which of the following is needed by surgeons during surgery?
Name a process by which an impure sample of naphthalene can be purified.
A gas can be best liquefied
A liquid is kept in an open china dish. A The evaporation of the liquid can be accelerated
The evaporation of a liquid occur only at
Which of the following is not a chemical reaction?
Which of the following is the nuclear fuel in the sun?
|
39 videos|110 docs|262 tests
|
|
39 videos|110 docs|262 tests
|

















