Chemistry: CUET Mock Test - 5 - CUET MCQ
30 Questions MCQ Test CUET UG Mock Test Series 2026 - Chemistry: CUET Mock Test - 5
Match list I with list II
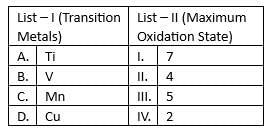
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
The metal from first transition series having positive 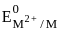 value :
value :
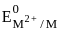 value :
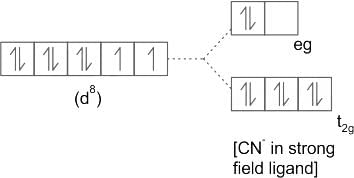
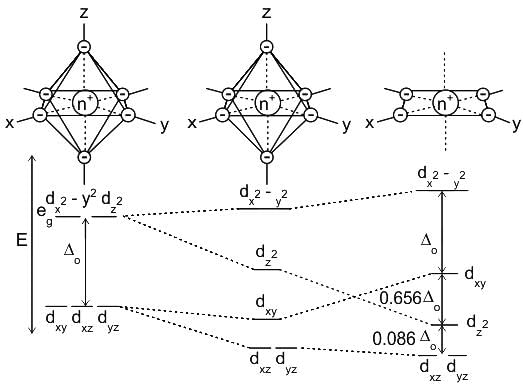
value :According to CFT (crystal field theory) [Ni(CN)4]2- has ___________.
Consider the following statements regarding electrochemical cells:
(A) A higher standard electrode potential indicates a greater tendency to gain electrons.
(B) A negative standard electrode potential indicates a higher tendency to gain electrons.
(C) In a galvanic cell, the electrons flow from the anode to the cathode through the external circuit.
(D) In an electrolytic cell, the cathode is the site of oxidation.
Choose the correct statements:
Consider the following statements regarding electrode potentials:
(A) Standard electrode potential is always measured under standard conditions (1 M concentration, 1 atm pressure, 25°C).
(B) The standard electrode potential of a cell is the sum of the cathode and anode potentials.
(C) A positive electrode potential indicates that the electrode is more likely to lose electrons.
(D) The standard electrode potential is a measure of the tendency of a species to gain electrons.
Choose the correct statements:
Consider the following statements about redox reactions:
(A) In a redox reaction, oxidation involves the loss of electrons.
(B) In a redox reaction, reduction involves the gain of electrons.
(C) A reducing agent is a substance that gains electrons and is reduced.
(D) An oxidizing agent is a substance that gains electrons and is reduced.
Choose the correct statements:
Consider the following statements about reaction rate:
(A) The rate of a reaction is always constant for a given temperature.
(B) The rate of a reaction is defined as the change in concentration of reactants or products per unit time.
(C) The rate of reaction increases with temperature.
(D) The rate of reaction is unaffected by the concentration of reactants.
Choose the correct statements:
1 mole of Al is deposited by X coulomb of electricity passing through aluminium nitrate solution. The number of moles of silver deposited by X coulomb of electricity from silver nitrate solution is-
A solution of Na2SO4 in water is electrolysed using Pt electrodes.The products at the cathode and anode are respectively -
A chemical reaction [2A] + [2B] + [C] → product follows the rate equation : then order of reaction is - [AIEEE-2002]
For the reaction system: 2NO(g) + O2(g) → 2NO2(g) volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with repect to NO, the rate of reaction will –
[AIEEE-2003]
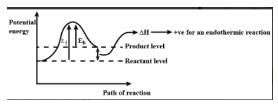
Consider an endothermic reaction X → Y with the activation energies Eb and Ef for the backward and forward reactions, respectively. In general
[AIEEE-2005]
Rate of reaction can be expressed by Arrhenius equation as k = Ae–E/RT , In this equation, E represents
[AIEEE 2006]
The first ionization energy of the d-block elements are?
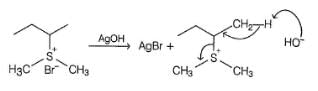
Which of the following gives 1-butene as the major product most easily on heating with AgOH?
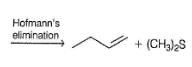
1-butene would be formed most easily in the following reaction when X is
Which of the following compound is most likely to follow E1 cb mechanism when treated with C2H5ONa in ethanol?
Which of the following statements are correct in case of the carbonyl bond between carbon and oxygen?
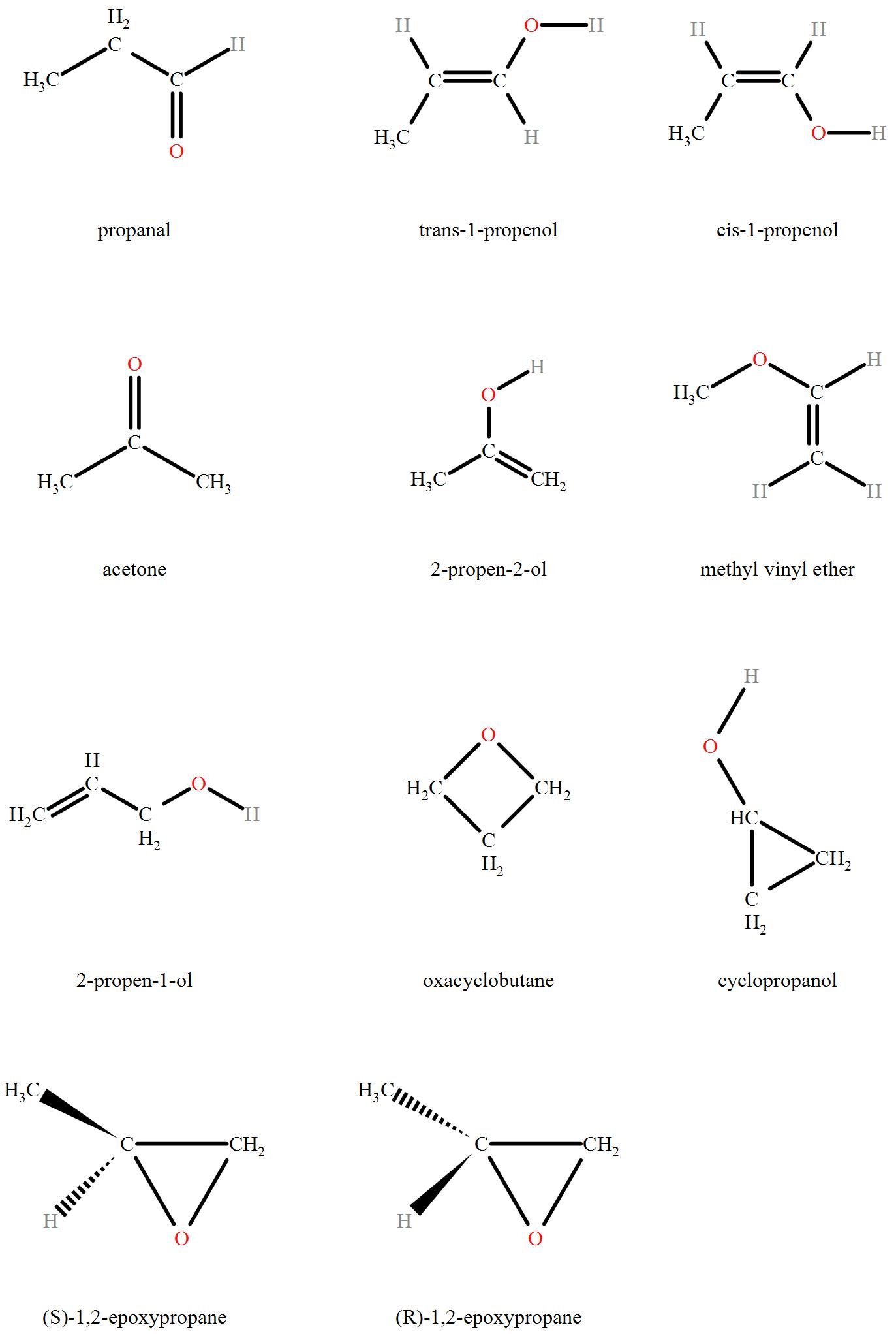
How many structural isomers can compound with molecular formula ‘C3H6O’ have?
Carbonyl compounds when oxidized easily to form carboxylic group involves
Which of the following polymer is stored in the liver of animals?
|
39 docs|145 tests
|


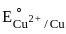 value in 3d series.
value in 3d series.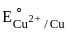 = 0.34 V
= 0.34 V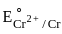 = –0.90 V
= –0.90 V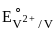 = –1.18 V
= –1.18 V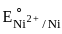 = = –0.25 V
= = –0.25 V


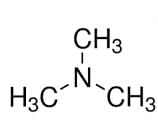 is a tertiary amine with IUPAC name:
is a tertiary amine with IUPAC name:










