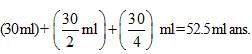Chemistry: Topic-wise Test- 5 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series - Updated 2026 Pattern - Chemistry: Topic-wise Test- 5
Which of the following species is not a pseudohalide ?
An orange solid (X) on heating , gives a colourless gas (Y) and a only green residue (Z). Gas (Y) treatment with Mg, produces a white solid substance...............
Only One Option Correct Type
Direction (Q, Nos. 1-9) This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which of the following will give a racemic mixture on reduction with NaBH4 followed by acid work-up?
Which of the following on reaction with excess of NaHSO3 in aqueous solution will give mixture of salts which can be separated into two fractions by fractional crystallisation?
Which is the most suitable reagent for the following transformation?
What is the final major product Y in the following reaction?
In the following reaction,
The major organic product is
Consider the following aldol condensation reaction,
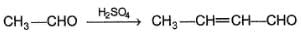
Q.
The nucleophile is
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The major organic product in the following reaction is
Which is the best hydride (H-) donor in the key step of Cannizaro reaction?
Only One Option Correct Type
Direction (Q. Nos. 1-7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which gives more than one amides on treatment with NH2OH followed by PCI5?
Which oximes on treatment with concentrated H2SO4 undergo rearrangement to give single amide?
What would be the major organic product in the following oxidation reaction?
Only One Option Correct Type
Direction (Q, Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
What is the major product of the following reaction?
Predict the major product of the following reaction
Which of the following polymer is stored in the liver of animals?
Sucrose (cane sugar) is a disaccharide. One molecule of sucrose on hydrolysis gives _________.
Proteins are found to have two different types of secondary structures viz. α-helix and β-pleated sheet structure. α-helix structure of protein is stabilised by :
The commonest disaccharide have the molecular formula:
Which of the following carbohydrates is called milk sugar?
In which structure of protein, the polypeptide chain forms all possible hydrogen bonds by twisting into right handed screw?
N205 → 2NO2 + O2
When N205 decompose, its t12 does not change with its changing pressure during the reaction, so which one is the correct representation for "pressure of 2NO2" vs lime° during the reaction when initial N205 is equals to Po
In a hypothetical reaction
A(aq)  2B(aq) + C(aq) (Ist order decomposition)
2B(aq) + C(aq) (Ist order decomposition)
'A' is optically active (dextro-rototory) while 'B' and 'C' are optically inactive but 'B' takes part in a titration reaction (fast reaction) with H202. Hence the progress of reaction can be monitored by measuring rotation of plane of plane polarised light or by measuring volume of H202 consumed in titration.
In an experiment the optical rotation was found to be 0 = 30° at t = 20 min and 8 =15° at t= 50 min. from start of the reaction. If the progress would have been monitored by titration method, volume of H202 consumed at t = 30 min. (from start) is 30 ml then volume of H202 consumed at t = 90 min will be:
At a certain temperature, the first order rate constant k1 is found to be smaller than the second order rate constant k2. If the energy of activation E1 of the first order reaction is greater than energy of activation E2 of the second order reaction, then with increase in temperature.
|
1 videos|19 docs|71 tests
|