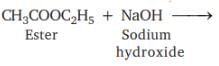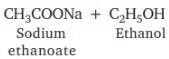Lakhmir Singh & Manjit Kaur Test: Carbon & its Compounds - SSS 1 MCQ
25 Questions MCQ Test - Lakhmir Singh & Manjit Kaur Test: Carbon & its Compounds
Which of the following will not decolourise bromine water?
Compounds made up of carbon and hydrogen only are called
Open-chain saturated hydrocarbons are called
The characteristic reaction of alkanes is
The major constituent of biogas is
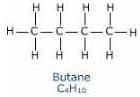
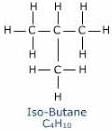
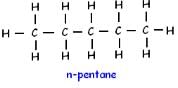
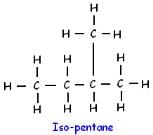
n-butane and isobutane are
Methane is a major constituent of
The major constituent of natural gas is
Ethanol on oxidation gives
The functional group present in carboxylic acids is
A dilute solution of ethanoic acid in water is called
Which of the following will undergo addition reactions?
Which of the following formula represents alkenes?
The general formula of cyclic alkanes is
A carboxylic group is present in
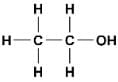
The functional group in an alcohol is
Which of the following will react with sodium metal?
Which of the following will give a pleasant smell of ester when heated with ethanol and a small quantity of sulphuric acid?
The functional group in aldehydes is
Ethanol on complete oxidation gives
Which class of organic compounds give effervescence with NaHCO3 solution ?
Carboxylic acids are obtained from alcohols by -
Soaps are prepared by alkaline hydrolysis of -
The functional group present in ethanol is
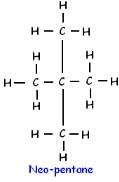
How many structural isomers are possible for pentane-