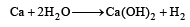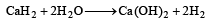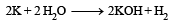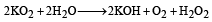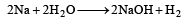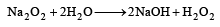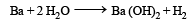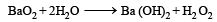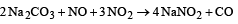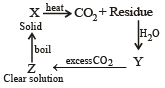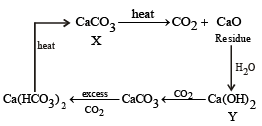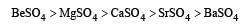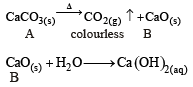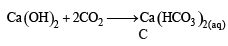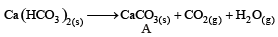31 Year NEET Previous Year Questions: The s-Block Elements (Not in NCERT) - NEET MCQ
30 Questions MCQ Test Inorganic Chemistry for NEET - 31 Year NEET Previous Year Questions: The s-Block Elements (Not in NCERT)
Which one of the following properties of alkali metals increases in magnitude as the atomic number rises ? [1989]
Wh ich of the following atoms will have the smallest size ? [1989]
Compared with the alkaline earth metals, the alkali metals exhibit [1990]
Washing soda has formula [1990]
Electronic configuration of calcium atom may be written as [1992]
Which of the following will react most vigorously with water
Which one of the following has minimum value of cation/anion ratio. [1993]
Which of the following has largest size ? [1993]
Which of the following is known as fusion mixture? [1994]
All the following substances react with water.The pair that gives the same gaseous product is [1994]
Among the following oxides, the one which is most basic is [1994]
Which of the following metal ions plays an important role in muscle contraction ? [1994]
Which of the following statement is false ? [1994]
Sodium is made by the electrolysis of a molten mixture of about 40% NaCl and 60% CaCl2 because [1995]
Identify the correct statement [1995]
Aqueous solution of sodium carbonate absorbs NO and NO2 to give [1996]
Which one is the correct statement with reference to solubility of MgSO4 in water? [1996]
Calcium is obtained by the [1997]
In crystals of which one of the following ionic compounds would you expect maximum distance between centres of cations and anions? [1998]
In which of the following processes, fused sodium hydroxide is electrolysed at a 330ºC temperature for extraction of sodium? [2000]
A solid compound ‘X’ on heating gives CO2 gas and a residue. The residue mixed with water forms ‘Y’. On passing an excess of CO2 through ‘Y’ in water, a clear solution ‘Z’, is obtained. On boiling ‘Z’, a compound ‘X’ is reformed. The compound ‘X’ is[2004]
The correct order of the mobility of the alkali metal ions in aqueous solutions is [2006]
The correct order of increasing thermal stability of K2CO3, MgCO3, CaCO3 and BeCO3 is[2007]
In which of the following the hydration energy is higher than the lattice energy? [2007]
The sequence of ioinic mobility in aqueous solution is : [2008]
The alkali metals form salt-like hydrides by the direct synthesis at elevated temperature. The thermal stability of these hydrides decreases in which of the following orders ? [2008]
Which of the following oxides is not expected to react with sodium hydroxide? [2009]
Which of the following alkaline earth metal sulphates has hydration enthalpy higher than the lattice enthalpy? [2010]
Property of the alkaline earth metals that increases with their atomic number : [2010]
The compound A on heating gives a colourless gas and a residue that is dissolved in water to obtain B. Excess of CO2 is bubbled through aqueous solution of B, C is formed which is recovered in the solid form. Solid C on gentle heating gives back A. The compound is [2010]
|
80 videos|112 docs|110 tests
|