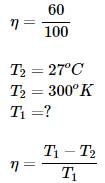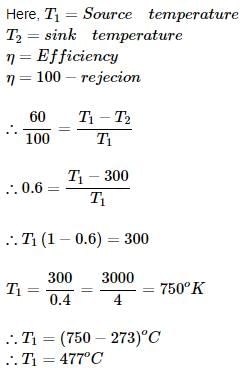Thermodynamics - 2 - Mechanical Engineering MCQ
20 Questions MCQ Test GATE Mechanical (ME) Mock Test Series 2025 - Thermodynamics - 2
A new temperature scale in degrees N is to be defined. The boiling and freezing points of water on this scale are 400° N and 100° N, respectively. What will be the reading on new scale corresponding to 60° C?
Measurement of temperature is based on which law of thermodynamics?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A steam turbine receives steam steadily at 10 bar with an enthalpy of 3000 kj/kg and discharges at 1 bar with an enthalpy of 2700 kj/kg. The work output is 250 kJ/kg. The changes in kinetic and potential energies are negligible. The heat transfer from the turbine casing to the surroundings is equal to
A system undergoes a change of state during which 80 kJ of heat is transferred to it and it does 60 kj of work. The system is brought back to its original state through a process during which 100 kj of heat is transferred to it. The work done by the system is
The internal energy of a certain system is a function of temperature alone and is given by the formula E = 25 + 0.25t kJ
If this system executes a process for which the work done by it per degree temperature increase is
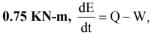
the heat interaction per degree temperature increase in kJ is
The air with enthalpy of 100 kJ/kg is compressed by an air compressor to a pressure and temperature at which its enthalpy becomes 200 kJ/kg. The loss of heat is 40 kJ/kg from the compressor as the air passes through it. Neglecting the change in kinetic and potential energies, the power required for an air mass flow of 0.5 kg/s is
Suppose 0.70 kg/s of air enters the compressor with a specific enthalpy of 290 kJ/kg and leaves it with specific enthalpy 450 kJ/kg. Velocities at inlet and exit are 6 m/s and 2 m/s, respectively. Assuming adiabatic process, what is power input to the compressor?
In a throttling process, which one of the following parameters remain constant?
A refrigerating machine working on reversed Carnot cycle takes out 2 kW per minute of heat from the system while between temperature limits of 300 K and 200 K. COP and power consumption of the cycle will be respectively
A reversible engine operates between temperatures T1 and T2. The energy rejected by this engine is received by a second reversible engine at temperature T2 and rejected to a reservoir at temperature T3. If the efficiencies of the engines are the same then the relationship between T1, T2 and T3 is given by
A Carnot engine operates between 327° C and 27° C. If the engine produces 300 kJ of work, what is the entiopy change during head addition?
The relation
ds = dQ/T
where s represents entropy, Q represents heat and T represents temperature (absolute), holds good in which one of the following processes?
An inventor says that his new concept of an engine, while working between temperature limits of 27° C and 327° C rejects 45% of heat absorbed from the source. His engine is then equivalent to which one of the following engines?
Three engines A, B and C operating on Carnot cycle use working substances as argon, oxygen and air, respectively. Which will have higher efficiency?
A series of combination of two Carnot engines operate between the temperatures of 180° C and 20°C. If the engine produces equal amount of work, then what is the intermediate temperature?
An engine working on Carnot cycle rejects 40% of absorbed heat from the source, while the sink temperature is maintained at 27°C, then what is the source temperature?
A reversible heat engine rejects 50% of the heat supplied during a cycle of operation. If this engine is reversed and operates as a heat pump, then what is its coefficient of performance?
When the lower temperature is fixed, COP of a refrigerating machine can be improved by
The heat absorbed or rejected during a polytropic process is equal to
|
29 docs|220 tests
|
|
29 docs|220 tests
|