31 Year NEET Previous Year Questions: Chemical Bonding & Molecular Structure - 3 - NEET MCQ
30 Questions MCQ Test - 31 Year NEET Previous Year Questions: Chemical Bonding & Molecular Structure - 3
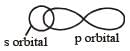
The angle between the overlapping of one s-orbital and one p-orbital is [1988]
Equilateral shape has [1988]
Which of the following molecule does not have a linear arrangement of atoms ? [1989]
Which of the following does not apply to metallic bond ?[1989]
In which one of the following molecules the central atom said to adopt sp2 hybridization?
H2O has a non zero dipole moment while BeF2 has zero dipole moment because [1989]
Among LiCl, BeCl2 BCl3 and CCl4, the covalent bond character follows the order [1990]
Which statement is NOT correct ? [1990]
Which one shows the strongest hydrogen bonding?
Linear combination of two hybridized orbitals belonging to two atoms and each having one electron leads to a [1990]
Which one of the following formulae does not correctly represent the bonding capacities of the two atoms involved ? [1990]
In compound X, all the bond angles are exactly 109°28; X is [1991]
Which of the following bonds will be most polar?
Which one of the following has the shortest carbon carbon bond length ? [1992]
Which structure is linear ? [1992]
Strongest hydrogen bond is shown by [1992]
Which of the following statements is not correct ? [1993]
Among the following which compound will show the highest lattice energy ? [1993]
Which one of the following is the correct order of interactions ? [1993]
Strongest bond is in between [1993]
Mark the incorrect statement in the following [1994]
The weakest among the following types of bonds is[1994]
Which of the following does not have a tetrahedral structure ? [1994]
Which of the following pairs will form the most stable ionic bond ? [1994]
Among the following orbital bonds, the angle is minimum between [1994]
Linus Pauling received the Nobel Prize for his work on [1994]
The boiling point of p-nitrophenol is higher than that of o-nitrophenol because [1994]
The distance between the two adjacent carbon atoms is largest in [1994]
Which of the following species is paramagnetic?
The BCl3 is a planar molecule whereas NCl3 is pyramidal because [1995]



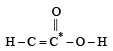
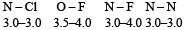
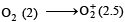 or decrease the bond order as in the conversion,
or decrease the bond order as in the conversion, 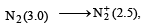 As a result, the bond energy may increase or decrease. thus, statement (b) is incorrect.
As a result, the bond energy may increase or decrease. thus, statement (b) is incorrect.










