Assertion & Reason Test: Thermodynamics - NEET MCQ
8 Questions MCQ Test - Assertion & Reason Test: Thermodynamics
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Combustion of all organic compounds is an exothermic reaction.
Reason (R) : The enthalpies of all elements in their standard state are zero.
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A) : Spontaneous process is an irreversible process and may be reversed by some external agency.
Reason (R) : Decrease in enthalpy is a contributory factor for spontaneity.
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A) : A liquid crystallises into a solid and is accompanied by decrease in entropy.
Reason (R) : In crystals, molecules organise in an ordered manner.
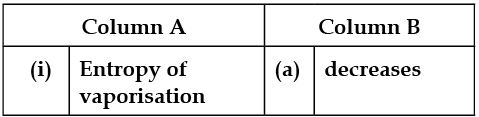
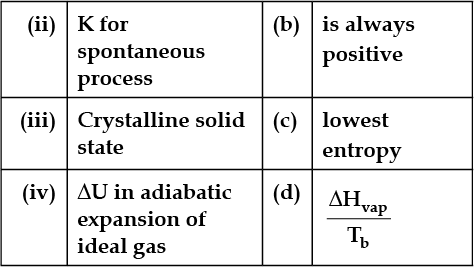
In the following questions, more than one correlation is possible between options of both columns.
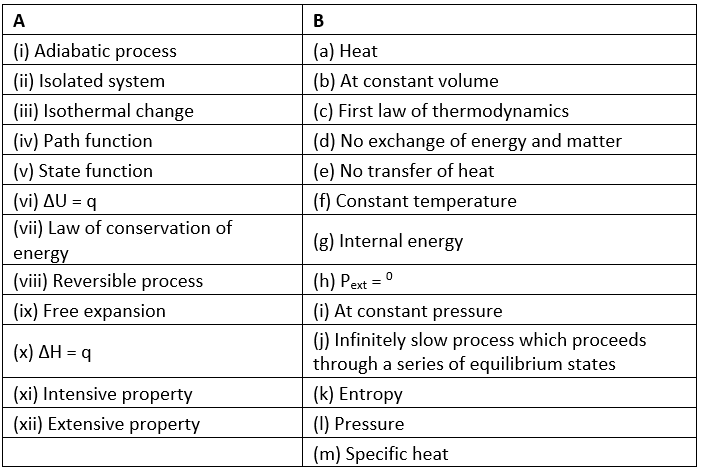
Match the following:
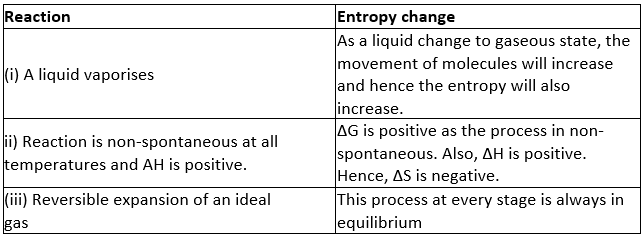
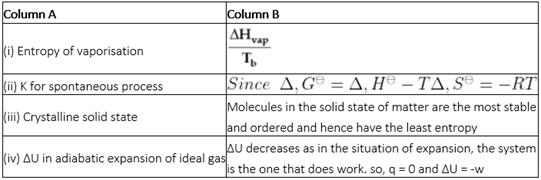
Match the following processes with entropy change:

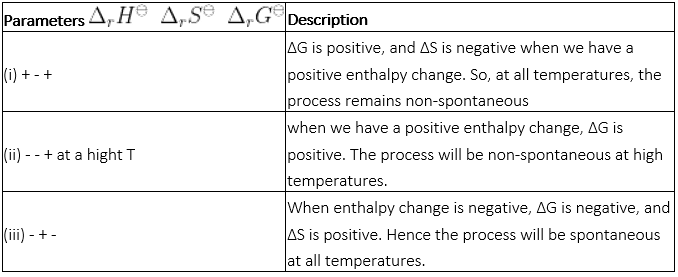
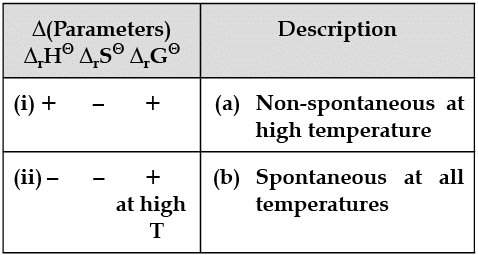
Match the following parameters with description for spontaneity :
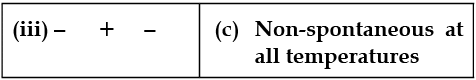
Assertion: The second law of thermodynamics states that the entropy of a closed or isolated system always increases. This means that all available energy is used up and there is no more potential for further useful work.
Reason: The system becomes disordered and also degraded.