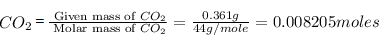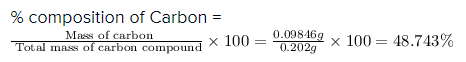Atoms And Molecules - Olympiad Level MCQ, Class 9 Science - Class 9 MCQ
30 Questions MCQ Test - Atoms And Molecules - Olympiad Level MCQ, Class 9 Science
The law of constant proportions was proposed by:
The weight of two elements which combine with each other are in the ratio of their :-
How many moles of oxygen atoms are present in one mole of acetic acid?
What is the number of particles in one mole of a substance?
How many atoms and how many gram atoms are there in 10 grams of calcium ?
Calculate the weight of 0.1 mole of sodium carbonate :-
How many number of moles are present in 540 g of glucose?
Formula for Aluminium Oxide is:
Chemical analysis of a carbon compound gave the following percentage composition by weight of the elements
present in it. Carbon = 10.06%, hydrogen = 0.84%, chlorine = 89.10%. Calculate the empirical formula of the compound :-
0.202 g of a carbon compound, on combustion, gave 0.361 g of carbon dioxide and 0.47 g of water. Calculate
the percentage composition of carbon :-
One mole of CO2 contains :
The percentage of nitrogen in ammonia is given by the expression :-
The empricial formula of a compound is CH2O. Its molecular weight is 90. Calculate the molecular formula of
the compound. (Atomic weight C = 12, H = 1, O = 16)
Calculate the weight of 2.5 mole of CaCO3 :-
How many gram atoms are present in 256 g of O2 ?
The molecular formula of potassium nitrate is ________.
The number of moles present in 20 grams of CaCO3 is :-
A hydrogen contain 90% of carbon and 10% hydrogen. The empirical formula of the compound is :-
An element has only one type of :-
The simplest formula of a compound having 50% of X(atomic weight 10) and 50% of Y(atomic weight 20) is :-
How many moles of glucose (C6H12O6) are present in 5.4 g ?
Calculate the number of gram atoms present in 8g of helium
How many moles are present in 5.3 g of anhydrous sodium carbonate ?
Calculate the number of moles in 60 gram NaOH.
Calculate the weight of nitrogen present in 0.5 moles of NH3.
Calculate the number of moles present in 7.3 g of HCl.
Calculate the weight in gram of 0.9 gram atoms of zinc.
Calculate the weight of 0.4 gram atoms of carbon
What is the weight of 3-gram atoms of sulphur?
16 gram of oxygen is equal to :-