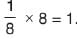BITSAT Chemistry Test - 5 - JEE MCQ
30 Questions MCQ Test BITSAT Mock Tests Series & Past Year Papers 2026 - BITSAT Chemistry Test - 5
The C−C bond length of the following molecules is in the order:
Which of the following statements is correct for electromeric effect?
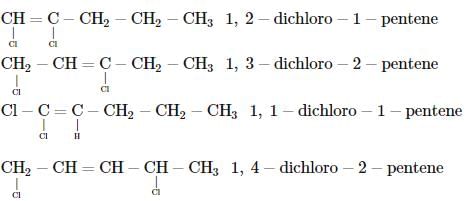
Which of the following does not show geometrical isomerism?
Mark the incorrect statement in nitrogen Kjeldahl's method of estimation
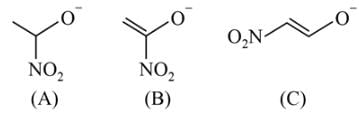
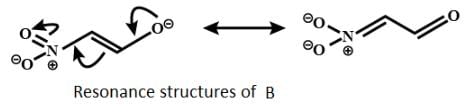
Which behaves both as a nucleophile as well as an electrophile?
The correct order of stability for the following alkoxides is:
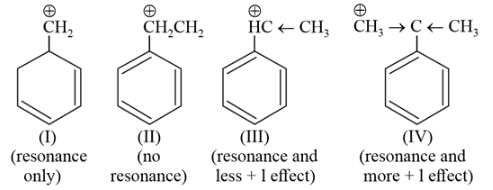
Arrange the following carbocations according to their stability order
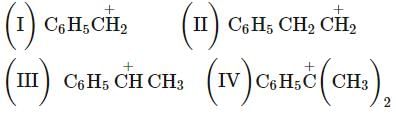
2 gm of a mixture of CO and CO2 on reaction with excess of I2O5 yields 2.54 gm of I2 . What would be the mass percentage of CO2 in the original mixture?
What is the maximum amount of BaSO4 that can be precipitated by mixing 0.5 M M BaCl2 with 1 M H2SO4?
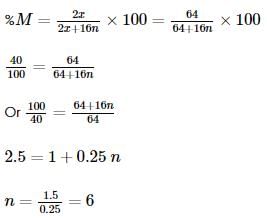
Two oxides of a metal contain 50% and 40% metal (M) respectively. If the formula of first oxide is MO2, the formula of second oxide will be
The atomic weights of two elements A and B are 40 u and 80 u, respectively. If xg of A contains y atoms, how many atoms are present in 2x g of B?
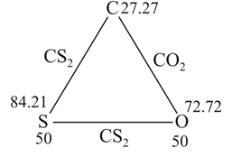
Carbon dioxide contains 27.27 % of carbon, carbon disulphide contains 15.79% of carbon and sulphur dioxide contains 50% of sulphur. This data is an agreement with:
The reactant which is entirely consumed in the reaction is known as limiting reagent. In the reaction,
2A+4B⟶3C+4D,
when 5 moles of A react with 6 moles of B then, calculate the amount of C formed.
The maximum number of moles of Ba3 (PO4)2 that can be formed if 2 mole BaCl2 is mixed with 1 mole Na3 PO4 is
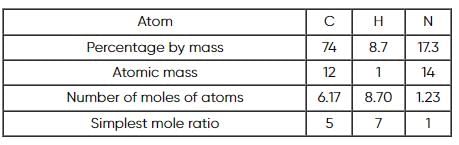
A compound is composed of 74% C , 8.7% H and 17.3% N by mass. If the molecular mass of the compound is 162, what is its molecular formula?
In three moles of ethane (C2H6), calculate the following
(i) Number of moles of carbon atoms.
(ii) Number of moles hydrogen atoms.
(iii) Number of molecule of ethane.
In nuclear reactor, the control rods are made up of cadmium because cadmium
How many electrons can be accommodated in a p- orbital?
In aqua-regia the ratio of cone. HNO3 and cone. HCI present is
Which of the following product is formed by the reaction of sulphurdioxide with chlorine in presence of sunlight ?
Avogadro’s number is the number of molecules present in
|
2 videos|19 docs|70 tests
|
|
2 videos|19 docs|70 tests
|


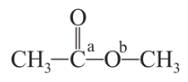
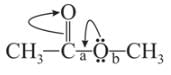

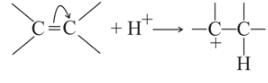
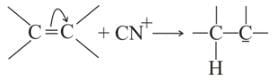
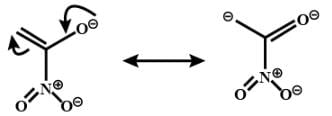
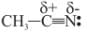 is a nucleophile due to the presence of a lone pair of electrons on N and is an electrophile due to the presence of a partial positive charge on C.
is a nucleophile due to the presence of a lone pair of electrons on N and is an electrophile due to the presence of a partial positive charge on C.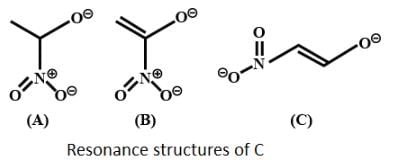
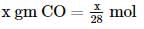
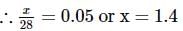
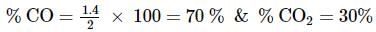

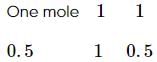
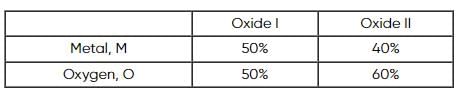
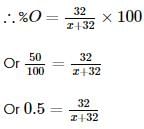
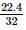 x 2 = 1.4L
x 2 = 1.4L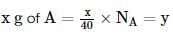
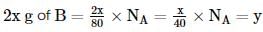
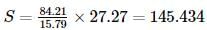
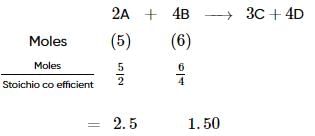
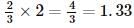 mole Na3 PO4
mole Na3 PO4