BITSAT Chemistry Test - 9 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 9
The hybridisation of carbon in diamond, graphite and acetylene is in the order of
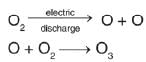
Ozone is prepared by passing silent electric discharge through oxygen. In this reaction
Which of the following compounds do not belong to lipids?
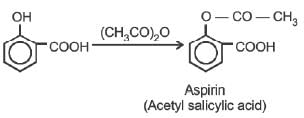
Aspirin is obtained by the reaction of salicylic acid with
A sudden large jump between the values of second and third ionization energies of an element would be associated with which of the following electronic configuration?
The oxidation of toluene to benzaldehyde by chromyl chloride is called
If acetyl chloride is reduced in the presence of BaS04 and Pd, then
A piece of magnesium was heated in the atmosphere of nitrogen. The compound formed after cooling and treating with water is
The right order of the solubility of sulphates of alkaline earth metals in water is
Which one of the following liberates oxygen on reacting with H2O?
A white precipitate is formed when gas A bubbled through slaked lime solution and the precipitate dissolves on prolonged bubbling of gas A. White precipitate reappears on heating the solution with evolution of gas B. Find the gases A and B.
Which alkali metal gives the least wavelength of light emitted in a flame?
Property of the alkaline earth metals that increases with their atomic number.
Sodium is heated in air at 300oC to form X. It absorbs CO2 and forms Na2CO3 and Y. Which of the following is Y?
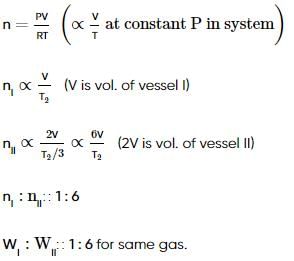
Two vessels connected by a valve of negligible volume. One container (I) has 2.8 g of N2 at temperature T1(K). The other container (II) is completely evacuated. The container (I) is heated to T2(K) while container (II) is maintained at T2 / 3 (K). Volume of vessel (I) is half that of vessel (II). If the valve is opened then what is the weight ratio of N2 in both vessel (WI / WII)?
According to Graham's law, at a given temperature the ratio of the rates of diffusion 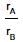 of gases A and B is given by (where, p and M are pressures and molecular weights of gases A and B respectively)
of gases A and B is given by (where, p and M are pressures and molecular weights of gases A and B respectively)
Which one of the following represents the graph between
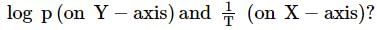
(p=vapour pressure of a liquid, T=absolute temperature)
The correct form of van der Waals' equation at low pressures is:
Which of the following relation is correct for one mole of an ideal gas,?
4.0 g of argon has pressure P at temperature T K in a vessel. On keeping the sample at 50° higher temperature, 0.8 g gas was given out to maintain the pressure P. The original temperature was
In which of the following mixtures, Dalton's law of partial pressure is not applicable? (Assume room temperature)
If 200 cm3 of gas at 1 atm is compressed to 50 cm3 at constant temperature, what will be the resultant pressure?
Decomposition of A in a rigid closed vessel takes place as A(g) → 3B(g), which follows 1st order kinetics. Reaction is started with an equimolar mixture of a gas A and an inert gas X. The initial pressure was 4 atm and at the end of 20 min, the total pressure is 6 atm. The time required for 75% reaction of A will be
Which of the following conditions show the polluted environment?
a. pH of rain water = 5.6
b. Eutrophication
c. Biochemical oxygen demand = 10 ppm
d. Amount of carbon dioxide in the atmosphere = 0.03%



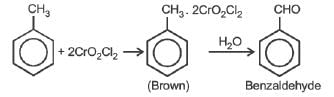
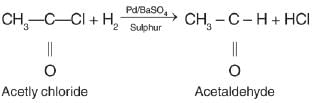
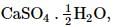 While the chemical formula of gypsum is CaSO4.2H2O
While the chemical formula of gypsum is CaSO4.2H2O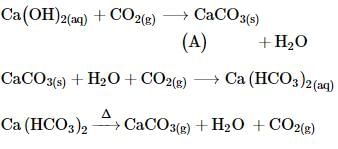
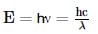
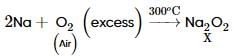
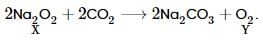
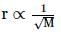
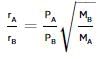
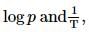 a straight line is obtained with negative slope.
a straight line is obtained with negative slope.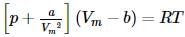
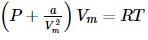
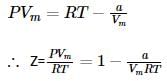
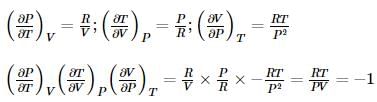
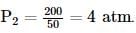
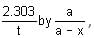 we get
we get










