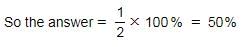Bihar STET Paper 1 Science Mock Test - 7 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar STET Paper 1 Science Mock Test - 7
The reaction, Zn + 2HCl → ZnCl2 + H2 is an example of:
Acids change the colour of methyl orange to:
What is the primary purpose of the chlor-alkali process?
Which industry commonly uses washing soda as a cleaning agent?
Which class of organic compounds give effervescence with NaHCO3 solution ?
Which of the following statements are true for acids ?
The no. of electrons in the valence shells of Sodium and Calcium repectively:
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that:
Which of the following has the smell of rotten egg?
Conversion of a liquid to a gas at all temperatures is called
Match the following with correct response.
Determine the mineral nutrient/element whose loss is compensated by growing a pulse crop between two cereal crops.
The mass number of an isotope of an element is 295, if its nucleus has 196 neutrons. What is its atomic number ?
Which of the following help in protecting the inner lining of the stomach from the harmful effect of hydrochloric acid?
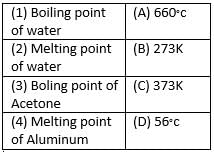
Match the following with correct response.
What conditions do proteins serve as respiratory substrates?
Which of the following approaches deals with explaining application of a particular concept first and its effects later on?
To assess the knowledge of the full syllabus in short time, the teacher should use
How should a teacher introduce a new topic in the class?
Continuous and Comprehensive Evaluation emphasizes:
Which of the following tools/methods does National Education Policy 2020 propose for assessment of children?
(i) Role plays
(ii) Group work
(iii) Portfolios
(iv) Projects
Who has been appointed as the Chief Executive Officer of BharatPe?
Which country has declared a health emergency due to escalating cases of dengue fever in 2024?
Urban sewage discharge and industrial effluents are the main cause of
How many biosphere reserves are there in India?
The time taken by 10 men to complete a work is the same as the time taken by 20 women to complete the same work. The efficiency of each woman is what percentage of the efficiency of each man?