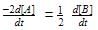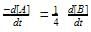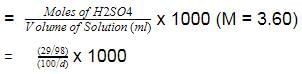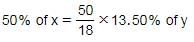Bihar STET Paper 2 Chemistry Mock Test - 10 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar STET Paper 2 Chemistry Mock Test - 10
When diazonium salt solution is treated with water at a temperature of 283 K it forms?
In Lyman series, shortest wavelength of H-atom appears at x m, then longest wavelength in Balmer series of He+ appear at
For a reaction 1/2 A→ 2B, rate of disappearance of ‘A’ related to the rate of appearance of ‘B’ by the expression -
[AIEEE 2008]
In which of the following molecules would you expect the N to N bond to be shortest ?
Passage
Consider the following isomers of [Co(NH3)4Br2]+. The black sphere represents Co, grey sphere represents NH3 and unshaded sphere represents Br.
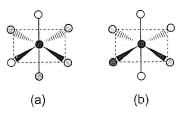
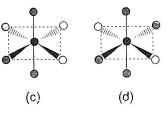
Q.
Which of the structures is identical?
Which one of the following is expected to exhibit optical isomerism?
(en = ethylenediamine)
Direction (Q. Nos. 14 -15) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d).
Based on the following thermochemical data of the given process, answer the questions.
Q. Bond energy of (C— C) bond is
What is the order of a reaction which has a rate expression ; Rate =
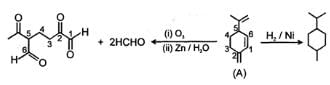
In the given sequence reaction which of the following is the correct structure of compounds A.
The density (in g mL-1) of a 3.60 M sulphuric acid solution that is 29% H2SO4(Molar mass = 98 g mol-1) by mass will be -
[AIEEE 2007]
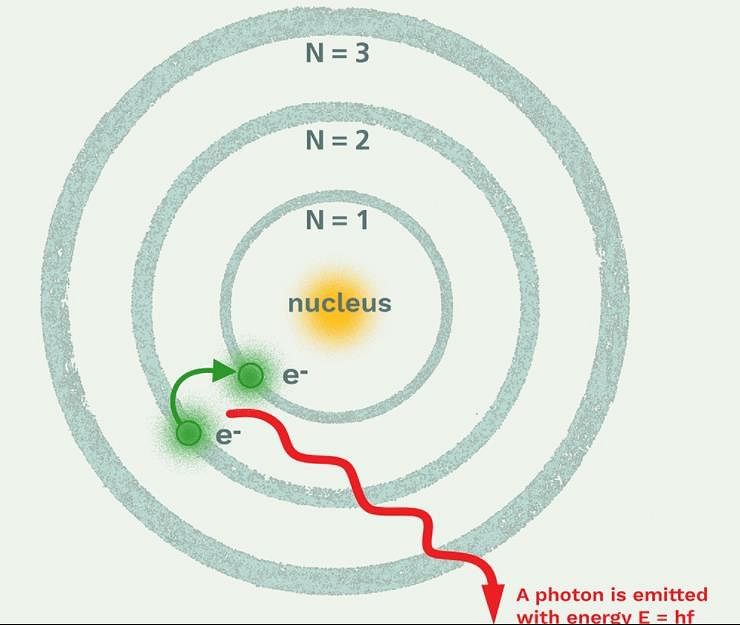
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?
In which of the following cases, entropy of I is larger than that of II?
Arrange the following in increasing order of boiling points.
I. 3 -methyl pentane
II. 3-chloropentane
III. 3-bromopentane
IV. 3,3-dichloropentane
A fully charged battery contains 500 mL of 5.00 M H2SO4. What is the concentration of H2SO4 in the battery after 6.0 A of current is drawn from the battery for 13.40 h?
Assuming (2s-2p) mixing is not operative, the paramagnetic species among the following is
[JEE Advanced 2014]
A complex of platinum, ammonia and chloride produces four ions per molecule in the solution. The structure consistent with the observation is :
With respect to electron affinity, which statement applies to the halogens?
In teaching-learning process, it is imperative that students ask questions in class. Why should a student be encouraged to ask questions in class?
The instructions provided by the teacher must be based on a child's
The third step in Herbart's inductive method of teaching is:
Mr. Rajesh aims to develop good speech habits in the learners. He should exploit
Who won the gold medal in the women's singles event at the 2023 World Badminton Championships?
What is the name of the world's largest iceberg that has recently begun an unusual journey?
Directions: Answer the following question by selecting the most appropriate option.
At the primary stage, assessments should consist of
Directions: Answer the following question by selecting the most appropriate option.
While discussing about friction, a teacher gave a number of examples to explain the concept. Some of the examples where friction is useful in our day-to-day lives as quoted by the teacher are given below:
A. We are able to write because of friction between the tip of the pen and the paper.
B. We are able to walk because of friction between our feet and the ground.
C. A ball thrown vertically upwards comes back to us due to friction.
D. We are able to stop a moving vehicle by applying brakes because of friction.
The correct examples are
If 18% of x is the same as 13.50% of y, 50% of x is the same as:



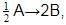 rate of disappearance of A is related to rate of appearance of B by the expression:
rate of disappearance of A is related to rate of appearance of B by the expression: