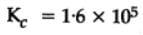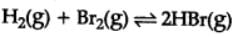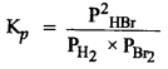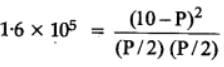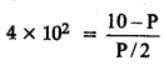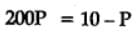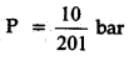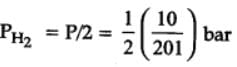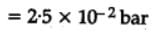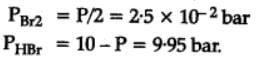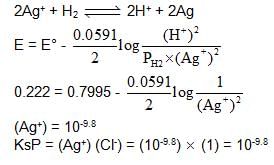Bihar STET Paper 2 Chemistry Mock Test - 3 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar STET Paper 2 Chemistry Mock Test - 3
The oxidation number of an element in a compound is evaluated on the basis of certain rules. Which of the following rules is not correct in this respect?
Which of the following can exhibit linkage isomerism?
Wave number of a spectral line for a given transition is x cm-1 for He+, then its value for Be3+ (isoelectronic of He+)for the same transition is
On moving down a group the number of valence electrons:
According to Bohr's theory, the angular momentum of an electron in 5th orbit is - [AIEEE 2006]
Ferric hydroxide is a negative sol, which of the following electrolyte will coagulate it most:
A binary liquid solution is prepared by mixing n-heptane and ethanol. Which one of the following statements is correct regarding the behaviour of the solution ?
[AIEEE 2009]
Uncertainty in position of a particle of 25 g in space is 10-5 m. Hence uncertainty in velocity (ms-1) is (Planck's constant h = 6.6 × 10-34 Js) [AIEEE- 2002]
What’s the name of the 109th element as per the nomenclature?
Volume occupied by one molecule of water (density = 1 g cm-3) is
The equilibrium constant for the following reaction, is 1.6 x 105 at 1024 K.
H2(g) + Br2(g) ⇌2HBr(g)
HBr (g)at 10.0 bar is introduced into a sealed container at 1024 K. Thus, partial pressure of H2(g)and Br2(g), together is
When 20 g of naphthoic acid (C11H8O2) is dissolved in 50 g of benzene, a freezing point depression of 2 K is observed. [Kf (benzene) = 1.72Kmol-1 kg]. The van’t Hoff factor (i) is
[IIT - JEE 2007]
How many stereoisomers exist for the compound 4-(1- propenyl) cyclohexane ?
Match the Column I with Column II and mark the correct option from the codes given below.
Following cell has EMF 0.7995 V.
Pt | H2 (1 atm) | HNO3 (1M) || AgNO3 (1M) | Ag
If we add enough KCl to the Ag cell so that the final Cl- is 1M. Now the measured emf of the cell is 0.222 V.
The Ksp of AgCl would be :
The major product of the following reaction is
Enthalpies of formation of CO(g) , CO2 (g) , N2O (g) and N2O4 (g) are -110, - 393, 81 and 9.7 kJ mol-1. Thus, ΔrU for the reaction at 298 K is,
Ca(HCO3)2 is strongly heated and after equilibrium is attained, temperature changed to 25° C.
Ca(HCO3)2(s)⇌CaO(s) + 2CO2 (g) + H2O(g)
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is
Which type of solids are held by weak dispersion forces?
Mr. Rathore uses a lot of audio-visual aids while teaching. The main reason for doing so is
Which term is often used interchangeably with the term 'Motivation'?
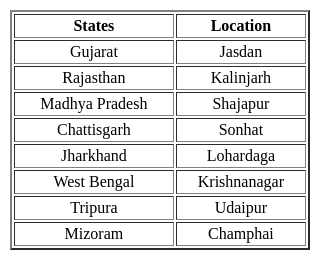
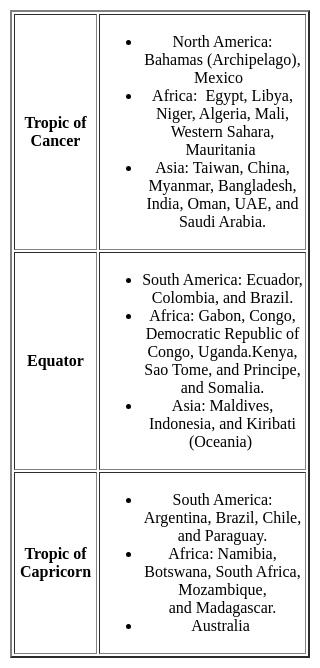
The Tropic of Cancer does NOT pass through which of the following state?
In the QS World University Rankings 2024, what is IIT Bombay's global ranking in engineering?
Which is the most deserted country in the world?