Chemistry - 2017 Past Year Paper - Chemistry MCQ
30 Questions MCQ Test IIT JAM Chemistry Past Year Papers - Chemistry - 2017 Past Year Paper
The correct order of the boiling points of the compounds is :
In the following Latimer diagram, the species that undergoes disproportionation reaction is :
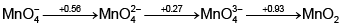
A yellow precipitate is formed upon addition of aqueous AgNO3 to a solution of :
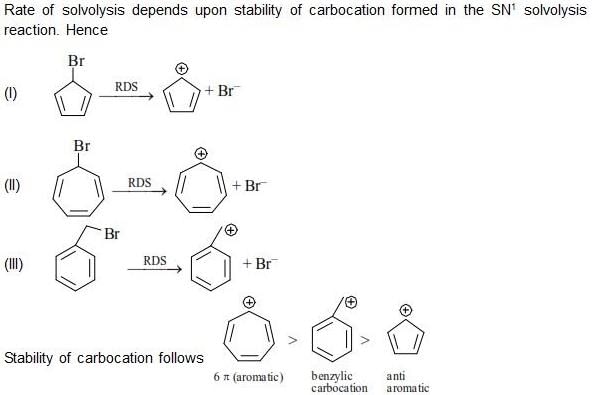
The correct order of rate of solvolysis for the following compounds is:
In the following sequence of reactions, the overall yield (%) of O is :
Catalytic hydrogenation of the following compound produces saturated hydrocarbon(s). The number of stereoisomer(s) formed is
The number of normal modes of vibration in naphthalene is :
The number of degrees of freedom of liquid water in equilibrium with ice is:
A straight line having a slope of -ΔUo / R is obtained in a plot between :
In a typical conductometric titration of a strong acid with a weak base, the curve resembles:
The coordination number of Al in crystalline AICl3 and liquid AICI3, respectively, is :
The homogeneous catalyst used in water- gas shift reaction is :
Nitrosyl ligand binds to d- metal atoms in linear and bent fashion and behaves, respectively, as:
The metal ion (M2+) in the following reaction is :
The correct order of wavelength of absorption (λmax) of the Cr-complexes is (en-ethylenediamine)
The correct order of enthalpy of hydration for the transition metal ions is :
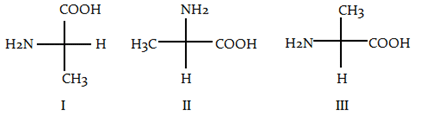
Which of the following is/are the S-enantiomer of alanine?
The number of proton NMR signals for the compounds P and Q, respectively is :
The correct set of reagents for the following conversion is :
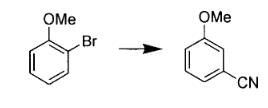
The major product S of the following reaction is :
In the following reaction, the major product T is :
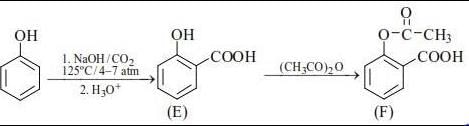
In the following reactions, the major product E and F, respectively, are :
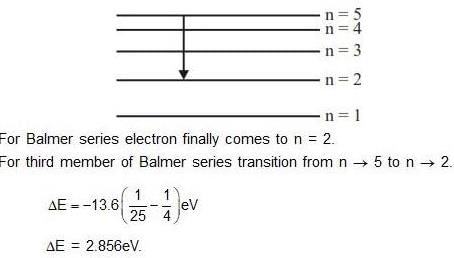
Ionization energy of hydrogen atom in ground state is 13.6 eV. The energy released (in eV) for third member of Balmer series is :
For a first order reaction A(g) → 2B(g) + C(g), the rate constant in terms of initial pressure (p0) and pressure at time t(pt), is given by :
For a particle in one- dimensional box of length L with potential energy V(x) = 0 and L > x > 0 and an acceptable wave function consistent with the boundary conditions is (a, b, c and d are constants)
|
5 docs|8 tests
|