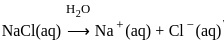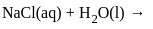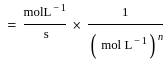Chemistry: CUET Mock Test - 2 - CUET MCQ
30 Questions MCQ Test - Chemistry: CUET Mock Test - 2
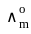 for NaCl, HCl and NaOAc are 126.4, 425.9 and 91.0 S cm2 mol-1 respectively. Calculate ∧° for HOAc
for NaCl, HCl and NaOAc are 126.4, 425.9 and 91.0 S cm2 mol-1 respectively. Calculate ∧° for HOAc
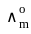 for NaCl, HCl and NaOAc are 126.4, 425.9 and 91.0 S cm2 mol-1 respectively. Calculate ∧° for HOAc
for NaCl, HCl and NaOAc are 126.4, 425.9 and 91.0 S cm2 mol-1 respectively. Calculate ∧° for HOAcThe products formed at cathode and anode by electrolysis of aqueous NaCl solution respectively are
Rate constant 'k' for a certain reaction is k = 2.3 × 10-5 L mol-1s-1. Order of the reaction is:
Which of the following statement is incorrect about the order of a reaction?
Why do colligative properties not significantly change with the addition of a small amount of a weak electrolyte to a solution?
What accurately describes how boiling point elevation and freezing point depression are related to solute concentration?
In the context of freezing point depression, why do roads get treated with salt in winter?
What is the effect of a non-volatile solute on the vapor pressure of a solution?
Osmotic pressure is a colligative property that demonstrates:
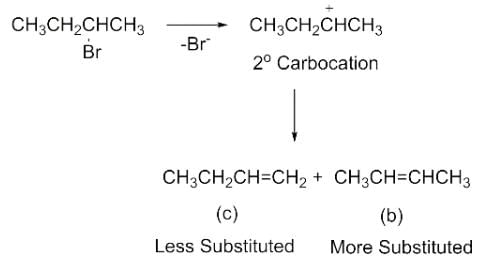
Elimination of bromine from 2-bromobutane results in the formation of
0.002 molal aqueous solution of an ionic compound [Co(NH3)5(N02)]CI freezes at - 0.00732° C. [Kf (H20) = 1.86° mol-1kg]. How many moles of ions does 1.0 mole of the salt produce on being dissolved in water?
Reduction of alkanenitriles with sodium and alcohol or LiAlH4 is called:
Nitro compounds are reduced by iron scrap and hydrochloric acid to yield one of the following compounds.
Which one of the following is used to increase blood pressure
Which of the following monosaccharides is a pentose-
Ring structure of glucose is due to formation of hemiacetal and ring formation between-
The inner transition elements are the elements in which the added electrons go to:
The second series of transition element starts with:
Knowing that the Chemistry of lanthanoids (Ln) is dominated by its +3 oxidation state, which of the following statements is incorrect ?
If zeise’s salt has the formula [Pt(C2H4)CI3]-. In this, platinum primary and secondary valency are
Which of the following isomeric alcohols is the most soluble in water?
Which of the following is necessary for the bromination of phenol?
Phenols are least soluble in which of the following?
Four compounds A, B, C and D having similar molecular masses were tested for their boiling points. It was found that compound C has the highest boiling point of all four. What is the compound C most likely to be?


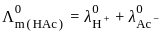
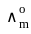 for NaCl, HCl and NaOAc are 126.4, 425.9 and 91.0 S cm2 mol-1
for NaCl, HCl and NaOAc are 126.4, 425.9 and 91.0 S cm2 mol-1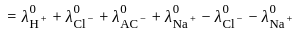
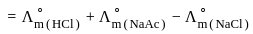
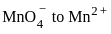 ?
?