Chemistry Test 1 - NEET/ AIIMS - Class 12 MCQ
30 Questions MCQ Test - Chemistry Test 1 - NEET/ AIIMS
According to molecular orbital theory, which is correct statement regarding O2?
The number of s and p-bonds present in pent-1-en-4-yne is
Which of the following is the correct electron dot structure of N2O molecule ?
The dipole moment of o, p and m-dichlorobenzene will be in the order
A molecule XY2 contains two s, two p bonds and one lone pair of electron in the valence shell of X. The arrangement of lone pair as well as bond pairs is
The boiling point of methanol is greater than methyl thiol because
Which one among the following is not paramagnetic ?
In which of the following molecules the central atom does not have sp3 hybridisation?
The shape of the compounds like ClF3, BrF5, XeF4 and SF4, respectively, are
Which of the following statements is correct about the molecular structure of boron trifluoride ?
Consider the statements
Then which of the following is correct ?
The volume of water to be added to 100 cm3 of 0.5 N H2SO4 to get decinormal concentration is
Which of the following has the most negative value of electron gain enthalpy ?
Which of the is known as the bridge element of 2nd group in Mendeleev’s table ?
Which of the following pairs are chemically dissimilar ?
The first ionization enthalpy of calcium is greater than that of potassium because for calcium,
The order of ionisation energies of the elements Li, Be, B, Na is
The property which is not common to both groups 1 and 17 elements in the periodic table is
Which of the following is correct increasing order of pH of the hydroxide solution of T, P and X ?
A photon of wavelength 5000 Å strikes a metal surface having work function of 2.20 eV. The kinetic energy of the emitted photo electron is
If the shortest wavelength of hydrogen atom in Lyman series is x, then longest wavelength in Paschen series of He+ is
If 20% nitrogen is present in a compound its minimum molecular weight will be
If the electron in a hydrogen atom drops from the n = 6 to the n = 4 level, the radiation emitted is in which series of lines in the spectrum of atomic hydrogen ?
The radius of hydrogen atom in the ground state is 0.53 Å. The radius of Li2+ ion (atomic number = 3) in a similar state is


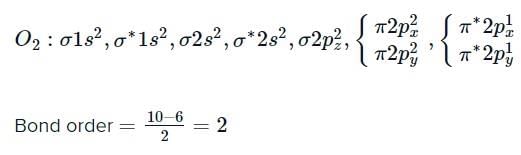
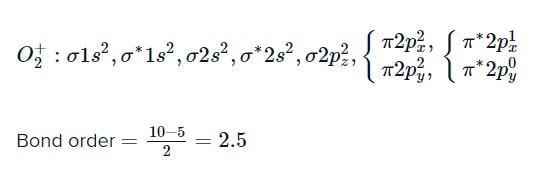
 both are paramagnetic, and bond order of
both are paramagnetic, and bond order of 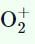 is greater than that of O2.
is greater than that of O2.












