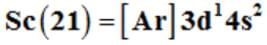Classification Of Elements & Periodicity In Properties - Practic Test (1) - Class 9 MCQ
25 Questions MCQ Test - Classification Of Elements & Periodicity In Properties - Practic Test (1)
Dmitri Mendeleev (1834-1907) and the German chemist, Lothar Meyer (1830-1895) proposed arranging elements in
Before the 4p orbital is filled, filling up of 3d orbitals becomes energetically favourable and there are 3d transition series of elements. This starts from scandium (Z = 21) which has the electronic configuration of .
The outer electronic configuration of f - Block elements are:
In terms of period and group where would you locate the element with Z=114 in the periodic table?
Of the following complex ions, which is diamagnetic in nature?
According to Mendeleev 'Periodic Law' states
An s orbital with one ml can accommodate x electrons, a p orbital with three ml y electrons, and a d orbital with five ml z electrons. The correct values of x, y and z are
The correct order for the increase in atomic radii is
An ionic compound is expected to have tetrahedral structure if - lies in the range of
The characteristic not related to alkali metal is
Mendeleev arranged elements in horizontal rows and vertical columns of a table in order of their increasing atomic weights in such a way that the elements with similar properties occupied
s-Block Elements comprise
The correct order for the decrease in atomic radii is
The relative tendency of an atom in a molecule to attract the shared pair of electrons towards itself is termed as its
The correct statement with regard to H+2 and H−2 is
Mendeleev relied on the similarities in the empirical formulas and properties of the compounds formed by the elements. He realized that some of the elements did not fit in with his scheme of classification if the order of atomic weight was strictly followed. He therefore
All the orbitals in the valence shell of the noble gases
One of the following has ns1 as its outermost electronic configuration
The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is:
The magnetic moment of a transition metal ion is 15−−√15 B.M. Therefore the number of unpaired electrons present in it is
Mendeleev proposed that some of the elements were still undiscovered and, therefore, left several gaps in the table. He left the gap under aluminium and a gap under silicon, and called these elements Eka-Aluminium and Eka-Silicon. These were later discovered and named
d-Block Elements are characterized by the
The order of increasing metallic character is:
Which of the following is not an actinoid?
An element with atomic number 21 is a